Abstract
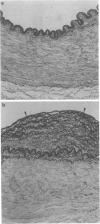
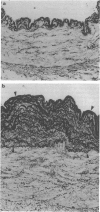
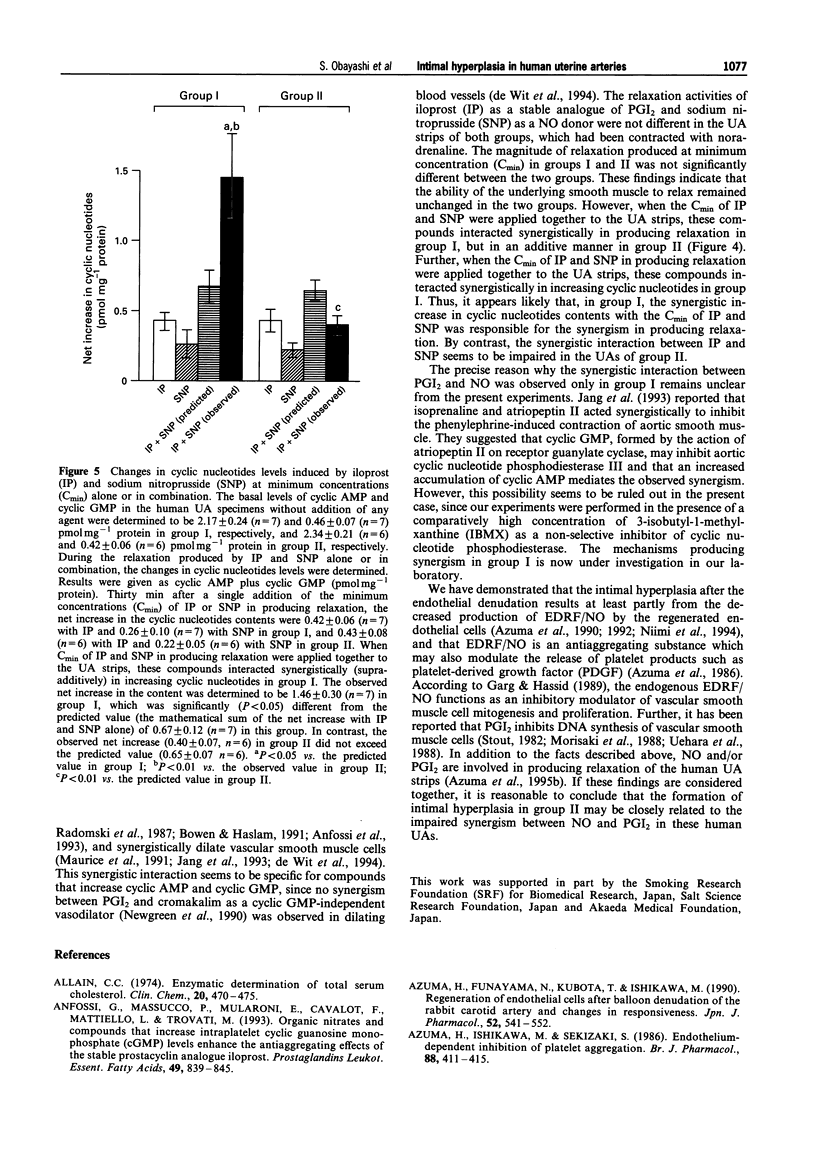
1. The present experiments were designed to investigate the mechanisms causing intimal hyperplasia in connection with the impaired synergism between prostaglandin I2 (PGI2) and nitric oxide (NO) in human uterine arteries (UAs). 2. In order to assess the magnitude of intimal hyperplasia, the intima:media ratio (%) was estimated with the aid of an image analyser. Human UAs were classified into two groups, I and II on the basis of the ratio and the degree of elastin deposition of histologically normal specimens. The intima:media ratio in group II was determined to be 38.9 +/- 7.7% (n = 6), which was significantly (P < 0.01) higher than that in group I (16.5 +/- 1.5%, n = 7). Less deposition of elastin was found in group I than in group II. 3. The relaxation activities of iloprost (IP) as a stable analogue of PGI2 and sodium nitroprusside (SNP) as a NO donor were not different between the two groups. When the minimum concentrations (Cmin) of IP and SNP in producing relaxation were applied together to the UA strips, these compounds interacted synergistically in group I. The observed relaxation (48.7 +/- 8.8%, n = 7) in this group was significantly (P < 0.01) greater than the predicted value of 18.8 +/- 3.1% (n = 7) (the mathematical sum of the relaxations caused by IP and SNP alone). By contrast, these agents interacted in an additive manner in group II. The observed relaxation (20.8 +/- 9.5%, n = 6) was not significantly different from the predicted value (18.6 +/- 2.4%, n = 6) in this group. 4. During the relaxation produced by the addition of IP and SNP alone or in combination, the changes in cyclic nucleotides (cyclic AMP and cyclic GMP) contents (pmol mg-1 protein) were assayed. When IP and SNP at Cmin were applied together to the UA strips, these compounds interacted synergistically in increasing cyclic nucleotides in group I. The observed net increase in the content was determined to be 1.46 +/- 0.30 (P < 0.05 vs. the predicted value of 0.67 +/- 0.12) in this group (n = 7). By contrast, the observed net increase (0.40 +/- 0.07, n = 6) did not exceed the predicted value (0.65 +/- 0.07, n = 6) in group II. 5. These results suggest that the formation of intimal hyperplasia in group II may be closely related to the impaired synergism between PGI2 and NO in the human UAs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- Anfossi G., Massucco P., Mularoni E., Cavalot F., Mattiello L., Trovati M. Organic nitrates and compounds that increase intraplatelet cyclic guanosine monophosphate (cGMP) levels enhance the antiaggregating effects of the stable prostacyclin analogue iloprost. Prostaglandins Leukot Essent Fatty Acids. 1993 Nov;49(5):839–845. doi: 10.1016/0952-3278(93)90207-d. [DOI] [PubMed] [Google Scholar]
- Azuma H., Funayama N., Kubota T., Ishikawa M. Regeneration of endothelial cells after balloon denudation of the rabbit carotid artery and changes in responsiveness. Jpn J Pharmacol. 1990 Apr;52(4):541–552. doi: 10.1254/jjp.52.541. [DOI] [PubMed] [Google Scholar]
- Azuma H., Ishikawa M., Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986 Jun;88(2):411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H., Niimi Y., Hamasaki H. Prevention of intimal thickening after endothelial removal by a nonpeptide angiotensin II receptor antagonist, losartan. Br J Pharmacol. 1992 Jul;106(3):665–671. doi: 10.1111/j.1476-5381.1992.tb14392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H., Niimi Y., Terada T., Hamasaki H. Accelerated endothelial regeneration and intimal hyperplasia following a repeated denudation of rabbit carotid arteries: morphological and immunohistochemical studies. Clin Exp Pharmacol Physiol. 1995 Oct;22(10):748–754. doi: 10.1111/j.1440-1681.1995.tb01930.x. [DOI] [PubMed] [Google Scholar]
- Azuma H., Obayashi S., Hamasaki H., Koyama T., Aso T. Role of endothelium in the human uterine arteries during normal menstrual cycle. Br J Pharmacol. 1995 Feb;114(4):902–908. doi: 10.1111/j.1476-5381.1995.tb13289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen R., Haslam R. J. Effects of nitrovasodilators on platelet cyclic nucleotide levels in rabbit blood; role for cyclic AMP in synergistic inhibition of platelet function by SIN-1 and prostaglandin E1. J Cardiovasc Pharmacol. 1991 Mar;17(3):424–433. doi: 10.1097/00005344-199103000-00011. [DOI] [PubMed] [Google Scholar]
- Fletcher M. J. A colorimetric method for estimating serum triglycerides. Clin Chim Acta. 1968 Nov;22(3):393–397. doi: 10.1016/0009-8981(68)90041-7. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma M., Satoh T., Takezawa J., Ui M. An ultrasensitive method for the simultaneous determination of cyclic AMP and cyclic GMP in small-volume samples from blood and tissue. Biochem Med. 1977 Dec;18(3):257–273. doi: 10.1016/0006-2944(77)90060-6. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989 Jul;65(1):1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Hattori K. Nitroarginine inhibits endothelium-derived relaxation. Jpn J Pharmacol. 1990 Jan;52(1):167–169. doi: 10.1254/jjp.52.167. [DOI] [PubMed] [Google Scholar]
- Kowaluk E. A., Seth P., Fung H. L. Metabolic activation of sodium nitroprusside to nitric oxide in vascular smooth muscle. J Pharmacol Exp Ther. 1992 Sep;262(3):916–922. [PubMed] [Google Scholar]
- Levin R. I., Weksler B. B., Jaffe E. A. The interaction of sodium nitroprusside with human endothelial cells and platelets: nitroprusside and prostacyclin synergistically inhibit platelet function. Circulation. 1982 Dec;66(6):1299–1307. doi: 10.1161/01.cir.66.6.1299. [DOI] [PubMed] [Google Scholar]
- Levitt M. A., Dryjski M., Tluczek J., Bjornsson T. D. Evaluation of a prostacyclin analog, iloprost, and a thromboxane A2 receptor antagonist, daltroban, in experimental intimal hyperplasia. Prostaglandins. 1991 Jan;41(1):1–6. doi: 10.1016/0090-6980(91)90099-2. [DOI] [PubMed] [Google Scholar]
- Lu J. K., Gilman D. P., Meldrum D. R., Judd H. L., Sawyer C. H. Relationship between circulating estrogens and the central mechanisms by which ovarian steroids stimulate luteinizing hormone secretion in aged and young female rats. Endocrinology. 1981 Mar;108(3):836–841. doi: 10.1210/endo-108-3-836. [DOI] [PubMed] [Google Scholar]
- Maurice D. H., Crankshaw D., Haslam R. J. Synergistic actions of nitrovasodilators and isoprenaline on rat aortic smooth muscle. Eur J Pharmacol. 1991 Jan 10;192(2):235–242. doi: 10.1016/0014-2999(91)90048-u. [DOI] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1978 Sep;30(3):293–331. [PubMed] [Google Scholar]
- Morisaki N., Kanzaki T., Motoyama N., Saito Y., Yoshida S. Cell cycle-dependent inhibition of DNA synthesis by prostaglandin I2 in cultured rabbit aortic smooth muscle cells. Atherosclerosis. 1988 Jun;71(2-3):165–171. doi: 10.1016/0021-9150(88)90140-2. [DOI] [PubMed] [Google Scholar]
- Newgreen D. T., Bray K. M., McHarg A. D., Weston A. H., Duty S., Brown B. S., Kay P. B., Edwards G., Longmore J., Southerton J. S. The action of diazoxide and minoxidil sulphate on rat blood vessels: a comparison with cromakalim. Br J Pharmacol. 1990 Jul;100(3):605–613. doi: 10.1111/j.1476-5381.1990.tb15854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi Y., Azuma H., Hirakawa K. Repeated endothelial removal augments intimal thickening and attenuates EDRF release. Am J Physiol. 1994 Apr;266(4 Pt 2):H1348–H1356. doi: 10.1152/ajpheart.1994.266.4.H1348. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Bolender R. P., Wight T. N., Clowes A. W. Heparin modulates the composition of the extracellular matrix domain surrounding arterial smooth muscle cells. Am J Pathol. 1990 Aug;137(2):313–330. [PMC free article] [PubMed] [Google Scholar]
- Stout R. W. Cyclic AMP: a potent inhibitor of DNA synthesis in cultured arterial endothelial and smooth muscle cells. Diabetologia. 1982 Jan;22(1):51–55. doi: 10.1007/BF00253870. [DOI] [PubMed] [Google Scholar]
- Strauss B. H., Chisholm R. J., Keeley F. W., Gotlieb A. I., Logan R. A., Armstrong P. W. Extracellular matrix remodeling after balloon angioplasty injury in a rabbit model of restenosis. Circ Res. 1994 Oct;75(4):650–658. doi: 10.1161/01.res.75.4.650. [DOI] [PubMed] [Google Scholar]
- Swedberg S. H., Brown B. G., Sigley R., Wight T. N., Gordon D., Nicholls S. C. Intimal fibromuscular hyperplasia at the venous anastomosis of PTFE grafts in hemodialysis patients. Clinical, immunocytochemical, light and electron microscopic assessment. Circulation. 1989 Dec;80(6):1726–1736. doi: 10.1161/01.cir.80.6.1726. [DOI] [PubMed] [Google Scholar]
- Tsujii T., Azuma H., Yamaguchi T., Oshima H. A possible role of decreased relaxation mediated by beta-adrenoceptors in bladder outlet obstruction by benign prostatic hyperplasia. Br J Pharmacol. 1992 Nov;107(3):803–807. doi: 10.1111/j.1476-5381.1992.tb14527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y., Ishimitsu T., Kimura K., Ishii M., Ikeda T., Sugimoto T. Regulatory effects of eicosanoids on thymidine uptake by vascular smooth muscle cells of rats. Prostaglandins. 1988 Dec;36(6):847–857. doi: 10.1016/0090-6980(88)90061-5. [DOI] [PubMed] [Google Scholar]
- de Wit C., von Bismarck P., Pohl U. Synergistic action of vasodilators that increase cGMP and cAMP in the hamster cremaster microcirculation. Cardiovasc Res. 1994 Oct;28(10):1513–1518. doi: 10.1093/cvr/28.10.1513. [DOI] [PubMed] [Google Scholar]