Abstract
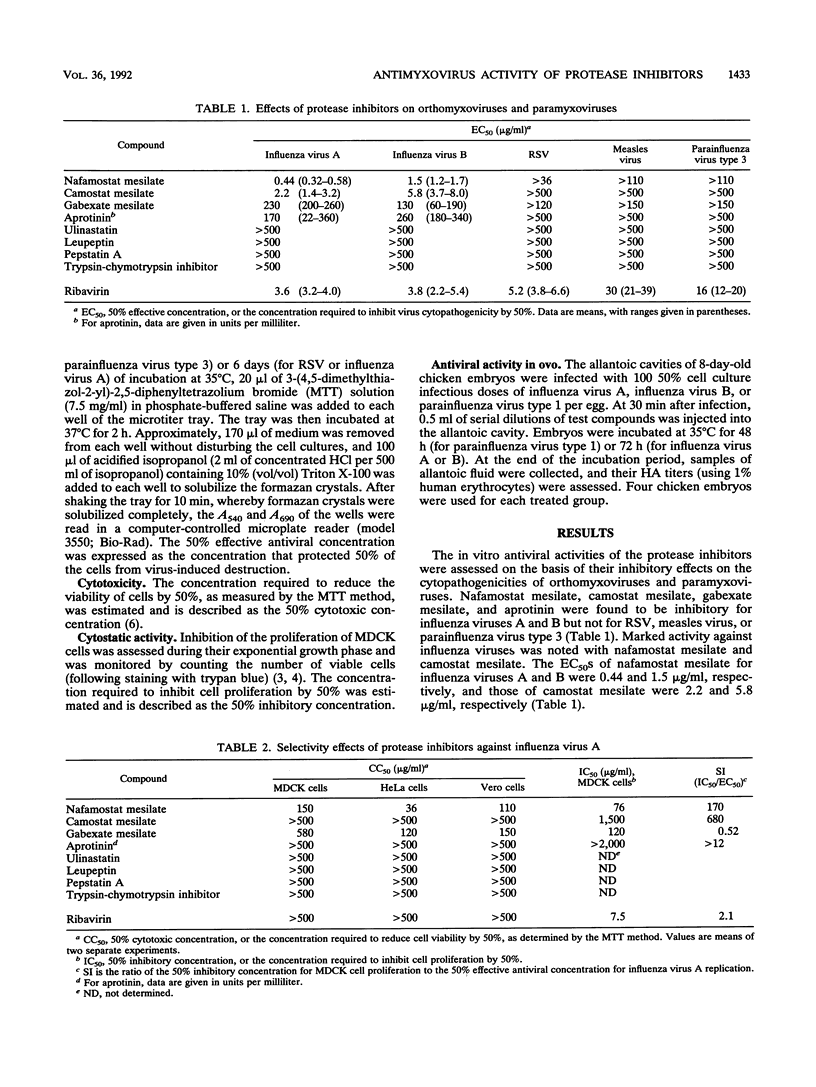
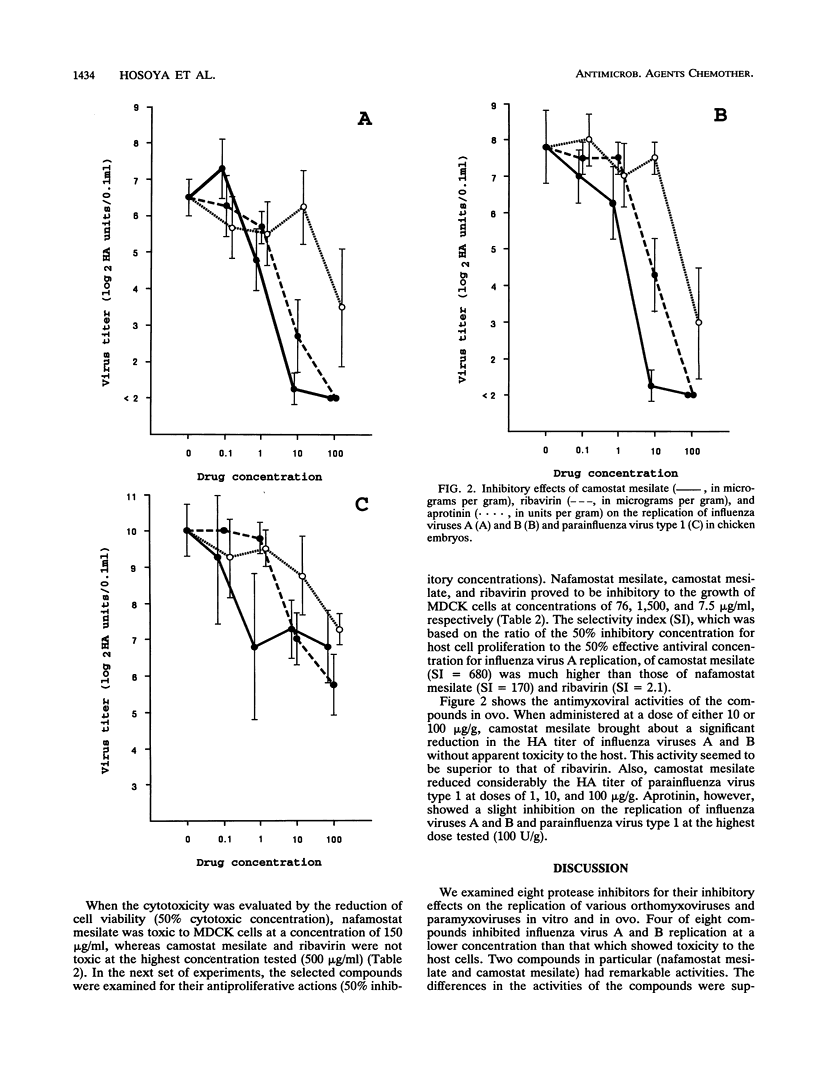
We studied the effects of eight protease inhibitors on the multicycle replications of various orthomyxoviruses and paramyxoviruses. Among the compounds, nafamostat mesilate, camostat mesilate, gabexate mesilate, and aprotinin, which are widely used in the treatment of pancreatitis, inhibited influenza virus A and B replication at concentrations that were significantly lower than their cytotoxic thresholds in vitro. None of the protease inhibitors had activity against respiratory syncytial virus, measles virus, or parainfluenza virus type 3 at the highest concentrations tested. Camostat mesilate was found to be the most selective inhibitor. Its 50% effective concentration for influenza virus A replication was 2.2 micrograms/ml, and the selectivity index, which was based on the ratio of the 50% inhibitory concentration for host cell proliferation to the 50% effective concentration for influenza virus A replication, was 680. When the in ovo antiviral activity of the compounds was tested by using chicken embryos, camostat mesilate at a dose of 10 micrograms/g markedly reduced the hemagglutinin titers of influenza viruses A and B.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berton M. T., Naeve C. W., Webster R. G. Antigenic structure of the influenza B virus hemagglutinin: nucleotide sequence analysis of antigenic variants selected with monoclonal antibodies. J Virol. 1984 Dec;52(3):919–927. doi: 10.1128/jvi.52.3.919-927.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Rose K., Kolakofsky D. Sequence determination of the Sendai virus fusion protein gene. J Gen Virol. 1985 Feb;66(Pt 2):317–331. doi: 10.1099/0022-1317-66-2-317. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Antiviral and antimetabolic activities of neplanocins. Antimicrob Agents Chemother. 1985 Jul;28(1):84–89. doi: 10.1128/aac.28.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Cools M., Balzarini J., Snoeck R., Andrei G., Hosoya M., Shigeta S., Ueda T., Minakawa N., Matsuda A. Antiviral activities of 5-ethynyl-1-beta-D-ribofuranosylimidazole-4- carboxamide and related compounds. Antimicrob Agents Chemother. 1991 Apr;35(4):679–684. doi: 10.1128/aac.35.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh B., Ogasawara T., Toyoda T., Inocencio N. M., Hamaguchi M., Nagai Y. An endoprotease homologous to the blood clotting factor X as a determinant of viral tropism in chick embryo. EMBO J. 1990 Dec;9(12):4189–4195. doi: 10.1002/j.1460-2075.1990.tb07643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya M., Shigeta S., Nakamura K., De Clercq E. Inhibitory effect of selected antiviral compounds on measles (SSPE) virus replication in vitro. Antiviral Res. 1989 Sep;12(2):87–97. doi: 10.1016/0166-3542(89)90072-7. [DOI] [PubMed] [Google Scholar]
- Kawana F., Shigeta S., Hosoya M., Suzuki H., De Clercq E. Inhibitory effects of antiviral compounds on respiratory syncytial virus replication in vitro. Antimicrob Agents Chemother. 1987 Aug;31(8):1225–1230. doi: 10.1128/aac.31.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Choppin P. W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975 Dec;68(2):440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D. Activation of precursors to both glycoporteins of Newcastle disease virus by proteolytic cleavage. Virology. 1977 Mar;77(1):125–134. doi: 10.1016/0042-6822(77)90412-3. [DOI] [PubMed] [Google Scholar]
- Numazaki Y., Shigeta S., Ishida N. Studies on parainfluenza virus infections among infants and children in Sendai. I. Isolation and identification methods of parainfluenza viruses. Jpn J Microbiol. 1968 Sep;12(3):275–281. doi: 10.1111/j.1348-0421.1968.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988 Aug;20(4):309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Shigeta S., Konno K., Yokota T., Nakamura K., De Clercq E. Comparative activities of several nucleoside analogs against influenza A, B, and C viruses in vitro. Antimicrob Agents Chemother. 1988 Jun;32(6):906–911. doi: 10.1128/aac.32.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya A., Tanaka N., Okuyama A. Inhibition of influenza virus A/WSN replication by a trypsin inhibitor, 6-amidino-2-naphthyl p-guanidinobenzoate. Biochem Biophys Res Commun. 1990 May 31;169(1):148–152. doi: 10.1016/0006-291x(90)91446-y. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Olmsted R. A., Venkatesan S., Coligan J. E., Collins P. L. Fusion glycoprotein of human parainfluenza virus type 3: nucleotide sequence of the gene, direct identification of the cleavage-activation site, and comparison with other paramyxoviruses. Virology. 1986 Jul 15;152(1):241–251. doi: 10.1016/0042-6822(86)90388-0. [DOI] [PubMed] [Google Scholar]
- Varsanyi T. M., Jörnvall H., Norrby E. Isolation and characterization of the measles virus F1 polypeptide: comparison with other paramyxovirus fusion proteins. Virology. 1985 Nov;147(1):110–117. doi: 10.1016/0042-6822(85)90231-4. [DOI] [PubMed] [Google Scholar]
- Verhoeyen M., Fang R., Jou W. M., Devos R., Huylebroeck D., Saman E., Fiers W. Antigenic drift between the haemagglutinin of the Hong Kong influenza strains A/Aichi/2/68 and A/Victoria/3/75. Nature. 1980 Aug 21;286(5775):771–776. doi: 10.1038/286771a0. [DOI] [PubMed] [Google Scholar]
- Zhirnov O. P., Ovcharenko A. V., Bukrinskaya A. G. Myxovirus replication in chicken embryos can be suppressed by aprotinin due to the blockage of viral glycoprotein cleavage. J Gen Virol. 1985 Jul;66(Pt 7):1633–1638. doi: 10.1099/0022-1317-66-7-1633. [DOI] [PubMed] [Google Scholar]


