Abstract
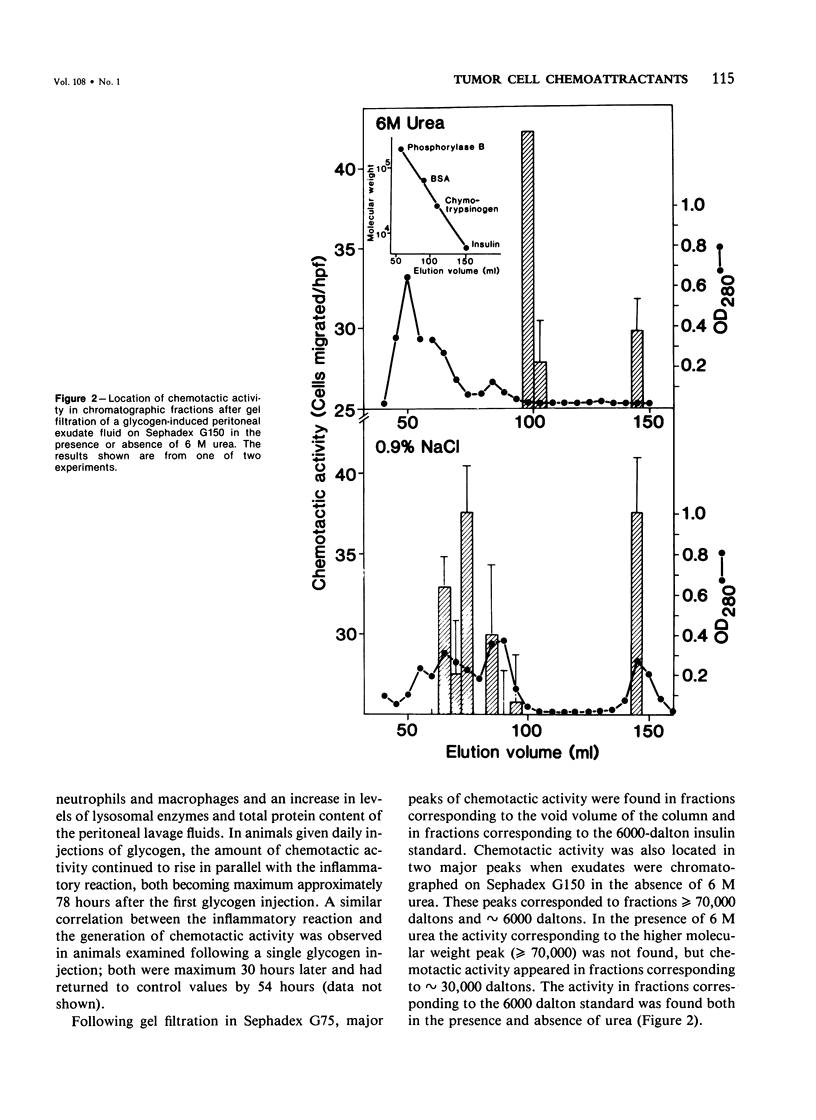
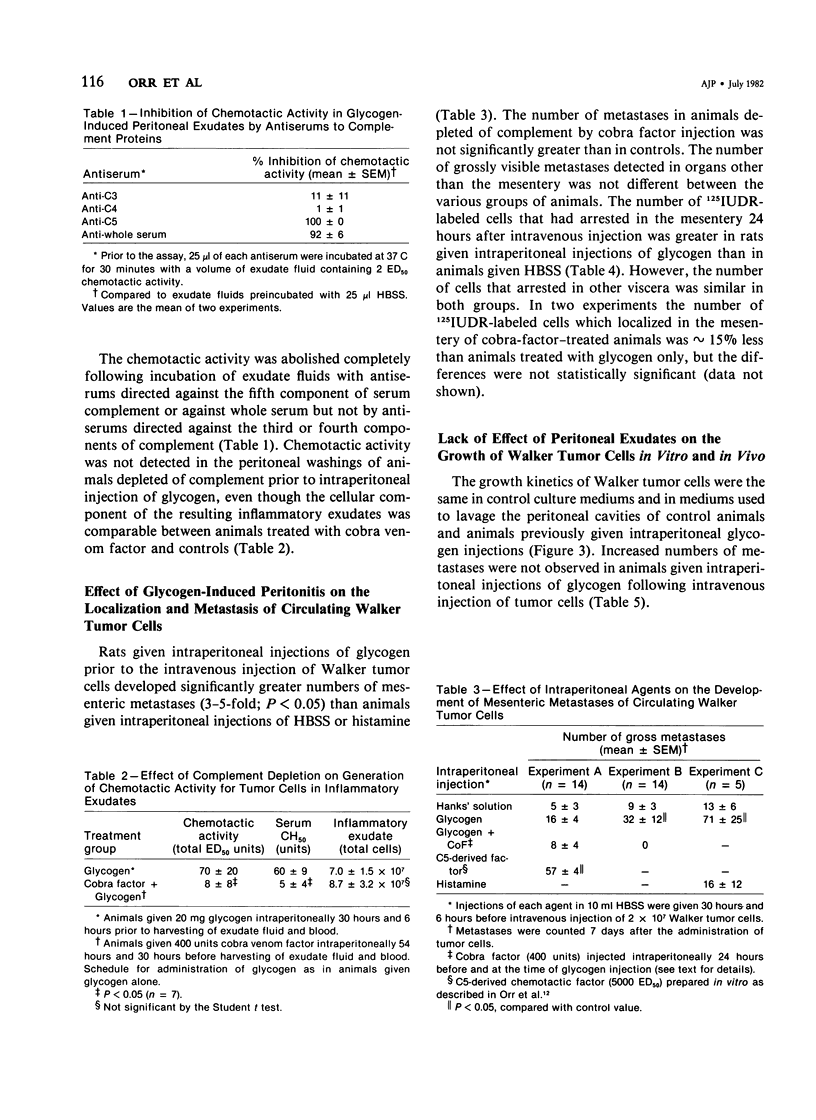
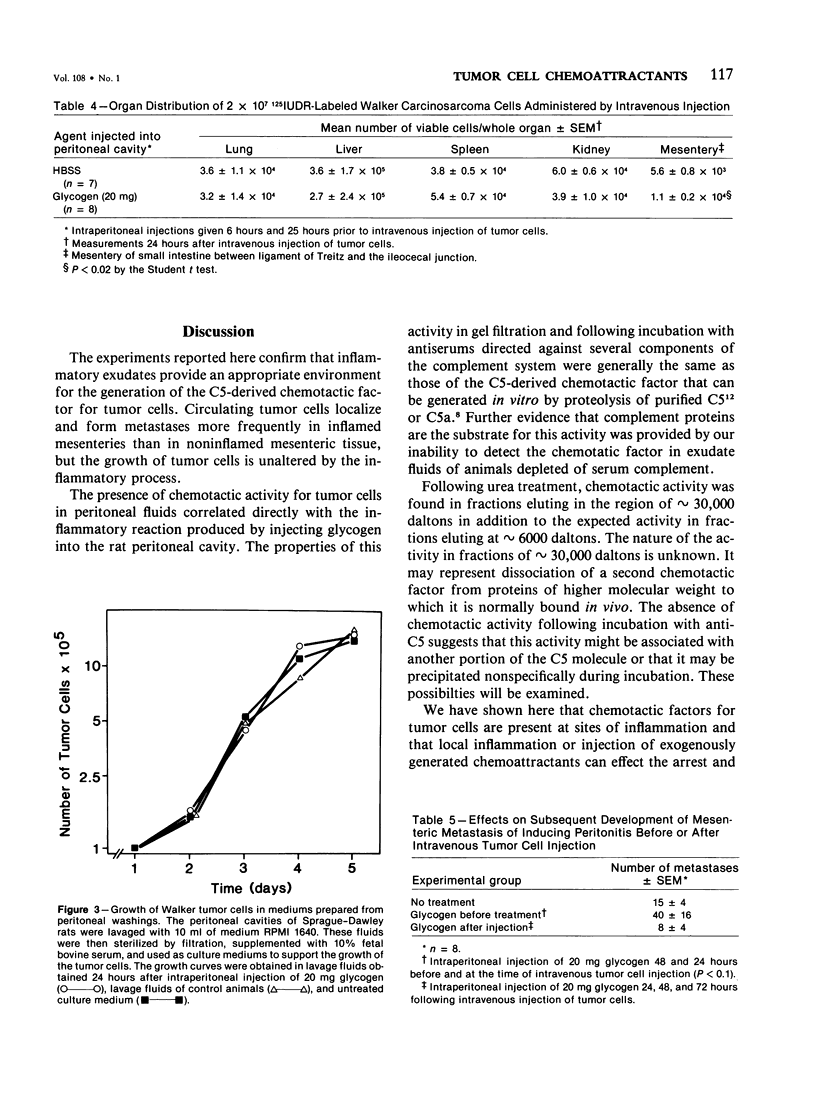
A chemotactic factor for tumor cells was found in inflammatory exudate fluids induced by giving intraperitoneal injections of glycogen to Sprague-Dawley rats. The quantity of chemotactic activity and the period of time during which it could be detected correlated with the inflammatory reaction, measured by the cellular composition of the exudates and their content of protein and lysosomal enzymes. In gel filtration the chemotactic factor behaved mainly as a molecule having a molecular weight of approximately 6000 daltons. Its biologic activity was blocked by antiserums directed against C5 but not by antiserums against C3 or C4. In these two respects, the factor generated in vivo has the same properties as a previously described chemotactic factor that can be generated in vitro by proteolysis of purified C5 or C5a. Chemotactic activity was not detected in the glycogen-induced peritoneal exudates of rats depleted of serum complement by cobra venom factor. Intravenously injected Walker tumor cells arrested and formed metastases in the mesenteries of rats with peritonitis in greater numbers than in normal controls, animals depleted of complement during the experimental period, or animals given intraperitoneal injections of the vasopermeability agent, histamine. The growth of tumor cells in vitro was not promoted by peritoneal exudate fluids, nor was the number of metastases on vivo greater than in negative controls, in animals in which peritonitis was induced 24 hours after the intravenous injection of tumor cells. It is argued that chemotactic mechanisms can contribute to the formation of metastases at sites of tissue injury.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGOSTINO D., CLIFFTON E. E. TRAUMA AS A CAUSE OF LOCALIZATION OF BLOOD-BORNE METASTASES: PREVENTIVE EFFECT OF HEPARIN AND FIBRINOLYSIN. Ann Surg. 1965 Jan;161:97–102. doi: 10.1097/00000658-196501000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B., Lockwood T., Morgan H. R. Surface biochemical changes accompanying primary infection with Rous sarcoma virus. II. Proteolytic and glycosidase activity and sublethal autolysis. Exp Cell Res. 1974 Jan;83(1):25–30. doi: 10.1016/0014-4827(74)90683-1. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Fisher B., Fisher E. R., Feduska N. Trauma and the localization of tumor cells. Cancer. 1967 Jan;20(1):23–30. doi: 10.1002/1097-0142(1967)20:1<23::aid-cncr2820200103>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Yoshida K., Ozaki T., Ushijima K. Chemotactic factor associated with invasion of cancer cells. Nature. 1970 Apr 11;226(5241):174–175. doi: 10.1038/226174a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lam W. C., Delikatny E. J., Orr F. W., Wass J., Varani J., Ward P. A. The chemotactic response of tumor cells. A model for cancer metastasis. Am J Pathol. 1981 Jul;104(1):69–76. [PMC free article] [PubMed] [Google Scholar]
- MUSA B. U., DOE R. P., SEAL U. S. PURIFICATION AND PROPERTIES OF HUMAN LIVER BETA-GLUCURONIDASE. J Biol Chem. 1965 Jul;240:2811–2816. [PubMed] [Google Scholar]
- O'Flaherty J. T., Ward P. A. Chemotactic factors and the neutrophil. Semin Hematol. 1979 Apr;16(2):163–174. [PubMed] [Google Scholar]
- OSLER A. G., STRAUSS J. H., MAYER M. M. Diagnostic complement fixation. I. A method. Am J Syph Gonorrhea Vener Dis. 1952 Mar;36(2):140–153. [PubMed] [Google Scholar]
- Orr F. W., Lam W. C., Delikatny E. J., Mokashi S., Varani J. Localization of intravenously injected tumor cells in the rat mesentery after intraperitoneal administration of chemotactic stimuli. Invasion Metastasis. 1981;1(4):239–247. [PubMed] [Google Scholar]
- Orr F. W., Varani J., Kreutzer D. L., Senior R. M., Ward P. A. Digestion of the fifth component of complement by leukocyte enzymes. Sequential generation of chemotactic activities for leukocytes and for tumor cells. Am J Pathol. 1979 Jan;94(1):75–83. [PMC free article] [PubMed] [Google Scholar]
- Orr W., Phan S. H., Varani J., Ward P. A., Kreutzer D. L., Webster R. O., Henson P. M. Chemotactic factor for tumor cells derived from the C5a fragment of complement component C5. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1986–1989. doi: 10.1073/pnas.76.4.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr W., Varani J., Gondex M. K., Ward P. A., Mundy G. R. Chemotactic responses of tumor cells to products of resorbing bone. Science. 1979 Jan 12;203(4376):176–179. doi: 10.1126/science.569363. [DOI] [PubMed] [Google Scholar]
- Orr W., Varani J., Ward P. A. Characteristics of the chemotactic response of neoplastic cells to a factor derived from the fifth component of complement. Am J Pathol. 1978 Nov;93(2):405–422. [PMC free article] [PubMed] [Google Scholar]
- Ozaki T., Yoshida K., Ushijima K., Hayashi H. Studies on the mechanisms of invasion in cancer. II. In vivo effects of a factor chemotactic for cancer cells. Int J Cancer. 1971 Jan 15;7(1):93–100. doi: 10.1002/ijc.2910070111. [DOI] [PubMed] [Google Scholar]
- Romualdez A. G., Jr, Ward P. A. A unique complement derived chemotactic factor for tumor cells. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4128–4132. doi: 10.1073/pnas.72.10.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G. B., Majno G. Acute inflammation. A review. Am J Pathol. 1977 Jan;86(1):183–276. [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Zvaifler N. J. Complement-derived leukotactic factors in inflammatory synovial fluids of humans. J Clin Invest. 1971 Mar;50(3):606–616. doi: 10.1172/JCI106531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass J. A., Varani J., Piontek G. E., Goff D., Ward P. A. Characteristics of the chemotactic factor-mediated cell swelling response of tumor cells. J Natl Cancer Inst. 1981 May;66(5):927–933. [PubMed] [Google Scholar]



