Abstract
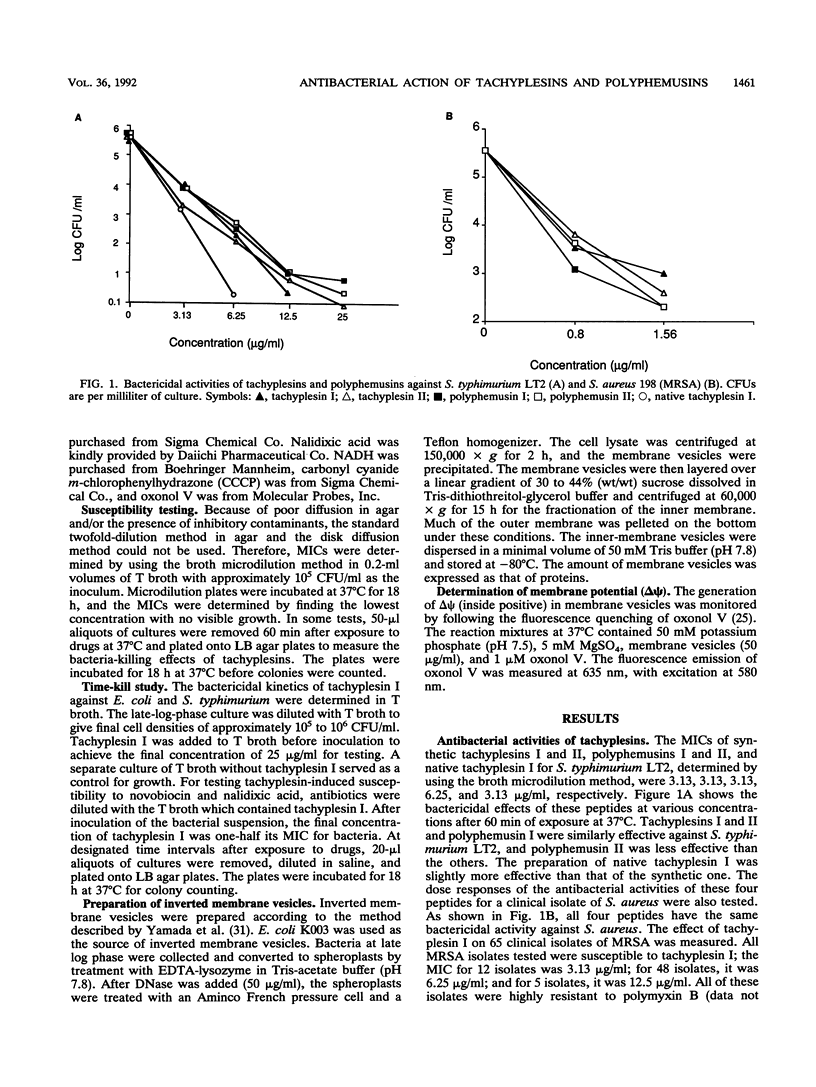
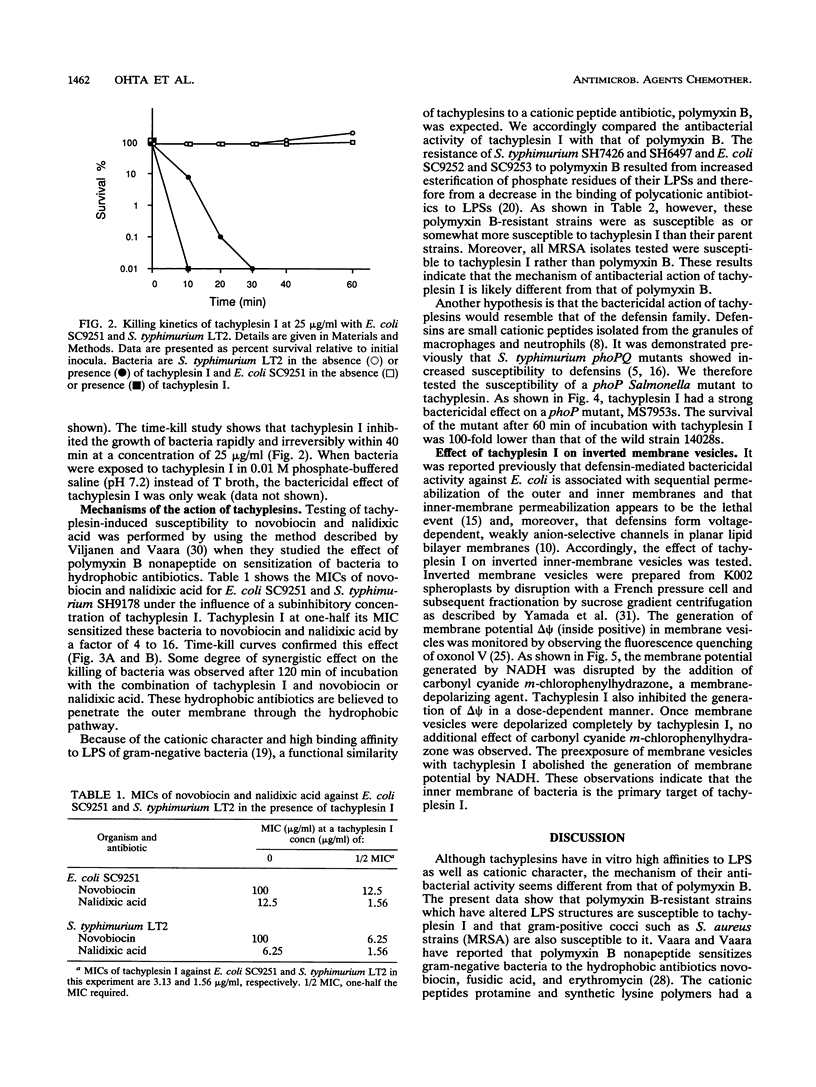
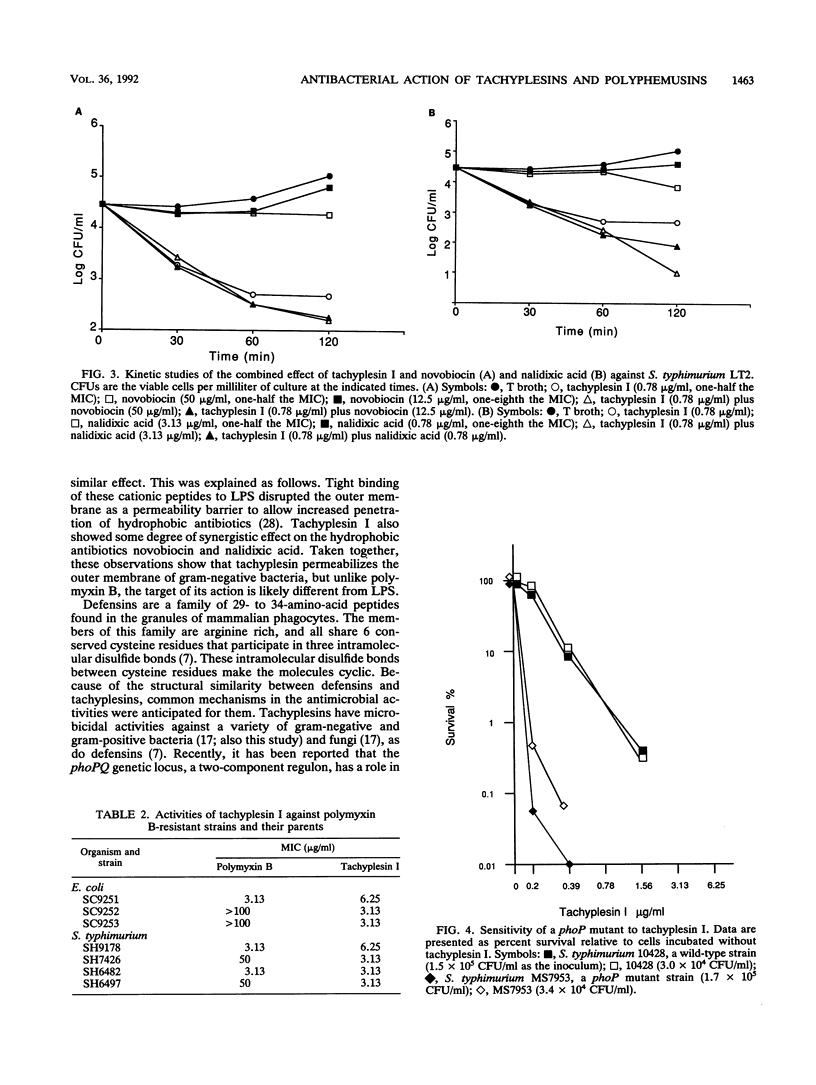
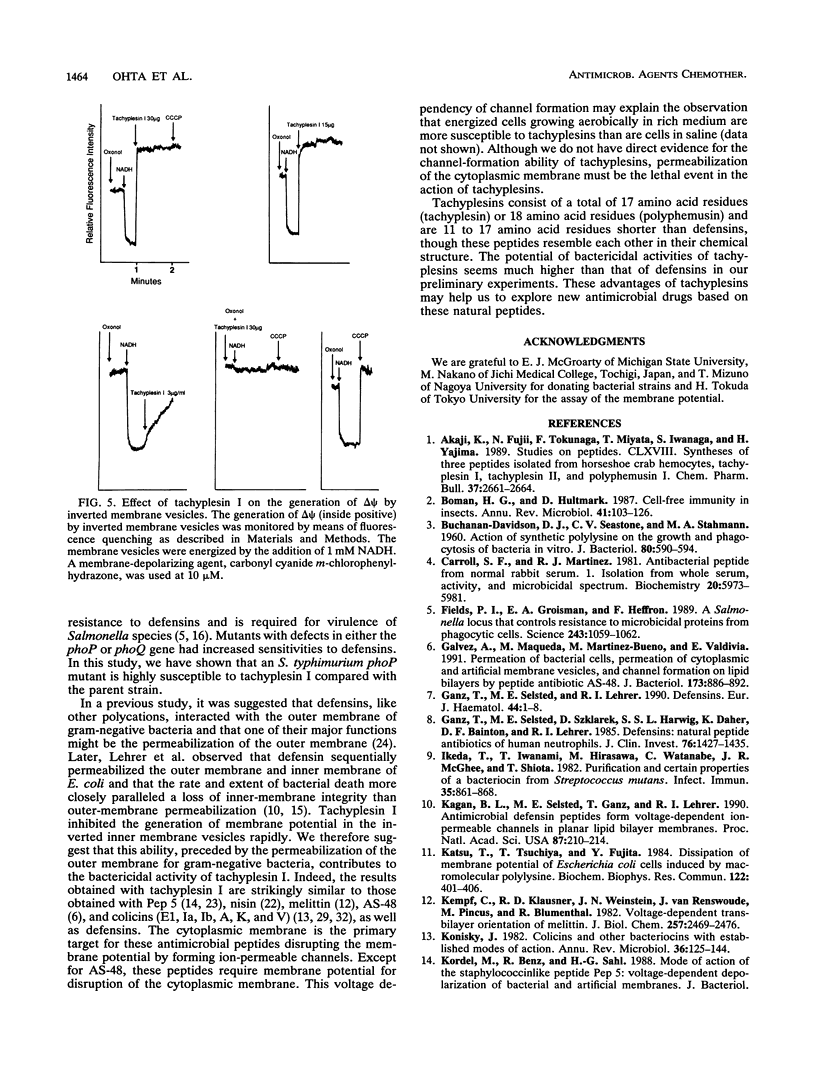
Tachyplesins I and II and polyphemusins I and II, cationic peptides isolated from the hemocytes of horseshoe crabs, show bactericidal activities with similar efficiencies for both gram-negative and gram-positive bacteria. Tachyplesin I inhibited bacterial growth irreversibly within 40 min. A subinhibitory concentration of tachyplesin I sensitized gram-negative bacteria to the bactericidal actions of novobiocin and nalidixic acid, although polymyxin B-resistant strains which have altered lipopolysaccharides were susceptible to tachyplesin I. This implies that tachyplesin permeabilizes the outer membrane and that the likely target of its action is outer membrane constituents other than lipopolysaccharides. On the other hand, a defensin-susceptible phoP strain of Salmonella typhimurium was also susceptible to tachyplesin I. Tachyplesin I rapidly depolarized the inverted inner-membrane vesicles of Escherichia coli. These results suggest that depolarization of the cytoplasmic membrane, preceded by the permeabilization of the outer membrane for gram-negative bacteria, is associated with tachyplesin-mediated bactericidal activity. The similarity between the actions of tachyplesin and those of defensin was discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boman H. G., Hultmark D. Cell-free immunity in insects. Annu Rev Microbiol. 1987;41:103–126. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- Buchanan-Davidson D. J., Seastone C. V., Stahmann M. A. ACTION OF SYNTHETIC POLYLYSINE ON THE GROWTH AND PHAGOCYTOSIS OF BACTERIA IN VITRO. J Bacteriol. 1960 Nov;80(5):590–594. doi: 10.1128/jb.80.5.590-594.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. F., Martinez R. J. Antibacterial peptide from normal rat serum. 1. Isolation from whole serum, activity, and microbicidal spectrum. Biochemistry. 1981 Oct 13;20(21):5973–5981. doi: 10.1021/bi00524a008. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Groisman E. A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Lehrer R. I. Defensins. Eur J Haematol. 1990 Jan;44(1):1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez A., Maqueda M., Martínez-Bueno M., Valdivia E. Permeation of bacterial cells, permeation of cytoplasmic and artificial membrane vesicles, and channel formation on lipid bilayers by peptide antibiotic AS-48. J Bacteriol. 1991 Jan;173(2):886–892. doi: 10.1128/jb.173.2.886-892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., Iwanami T., Hirasawa M., Watanabe C., McGhee J. R., Shiota T. Purification and certain properties of a bacteriocin from Streptococcus mutans. Infect Immun. 1982 Mar;35(3):861–868. doi: 10.1128/iai.35.3.861-868.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan B. L., Selsted M. E., Ganz T., Lehrer R. I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci U S A. 1990 Jan;87(1):210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsu T., Tsuchiya T., Fujita Y. Dissipation of membrane potential of Escherichia coli cells induced by macromolecular polylysine. Biochem Biophys Res Commun. 1984 Jul 18;122(1):401–406. doi: 10.1016/0006-291x(84)90489-3. [DOI] [PubMed] [Google Scholar]
- Kempf C., Klausner R. D., Weinstein J. N., Van Renswoude J., Pincus M., Blumenthal R. Voltage-dependent trans-bilayer orientation of melittin. J Biol Chem. 1982 Mar 10;257(5):2469–2476. [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Barton A., Daher K. A., Harwig S. S., Ganz T., Selsted M. E. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest. 1989 Aug;84(2):553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Pulkkinen W. S., Selsted M. E., Mekalanos J. J. Characterization of defensin resistance phenotypes associated with mutations in the phoP virulence regulon of Salmonella typhimurium. Infect Immun. 1990 Nov;58(11):3706–3710. doi: 10.1128/iai.58.11.3706-3710.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T., Tokunaga F., Yoneya T., Yoshikawa K., Iwanaga S., Niwa M., Takao T., Shimonishi Y. Antimicrobial peptides, isolated from horseshoe crab hemocytes, tachyplesin II, and polyphemusins I and II: chemical structures and biological activity. J Biochem. 1989 Oct;106(4):663–668. doi: 10.1093/oxfordjournals.jbchem.a122913. [DOI] [PubMed] [Google Scholar]
- Muta T., Fujimoto T., Nakajima H., Iwanaga S. Tachyplesins isolated from hemocytes of Southeast Asian horseshoe crabs (Carcinoscorpius rotundicauda and Tachypleus gigas): identification of a new tachyplesin, tachyplesin III, and a processing intermediate of its precursor. J Biochem. 1990 Aug;108(2):261–266. doi: 10.1093/oxfordjournals.jbchem.a123191. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Furunaka H., Miyata T., Tokunaga F., Muta T., Iwanaga S., Niwa M., Takao T., Shimonishi Y. Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure. J Biol Chem. 1988 Nov 15;263(32):16709–16713. [PubMed] [Google Scholar]
- Peterson A. A., Fesik S. W., McGroarty E. J. Decreased binding of antibiotics to lipopolysaccharides from polymyxin-resistant strains of Escherichia coli and Salmonella typhimurium. Antimicrob Agents Chemother. 1987 Feb;31(2):230–237. doi: 10.1128/aac.31.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. S., Bhargava P. M. Seminalplasmin--an antimicrobial protein from bovine seminal plasma which acts in E. coli by specific inhibition of rRNA synthesis. Nature. 1979 Jun 21;279(5715):725–728. doi: 10.1038/279725a0. [DOI] [PubMed] [Google Scholar]
- Ruhr E., Sahl H. G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985 May;27(5):841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl H. G. Influence of the staphylococcinlike peptide Pep 5 on membrane potential of bacterial cells and cytoplasmic membrane vesicles. J Bacteriol. 1985 May;162(2):833–836. doi: 10.1128/jb.162.2.833-836.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer J. G., Martin N. L., Hancock R. E. Interaction of macrophage cationic proteins with the outer membrane of Pseudomonas aeruginosa. Infect Immun. 1988 Mar;56(3):693–698. doi: 10.1128/iai.56.3.693-698.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherman D., Henry J. P. Oxonol-V as a probe of chromaffin granule membrane potentials. Biochim Biophys Acta. 1980 Jun 20;599(1):150–166. doi: 10.1016/0005-2736(80)90064-4. [DOI] [PubMed] [Google Scholar]
- Selsted M. E., Szklarek D., Lehrer R. I. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun. 1984 Jul;45(1):150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm D. R., Rosenthal K. S., Swanson P. E. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations sensitize enteric bacteria to antibiotics. Antimicrob Agents Chemother. 1983 Jul;24(1):107–113. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varley J. M., Boulnois G. J. Analysis of a cloned colicin Ib gene: complete nucleotide sequence and implications for regulation of expression. Nucleic Acids Res. 1984 Sep 11;12(17):6727–6739. doi: 10.1093/nar/12.17.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljanen P., Vaara M. Susceptibility of gram-negative bacteria to polymyxin B nonapeptide. Antimicrob Agents Chemother. 1984 Jun;25(6):701–705. doi: 10.1128/aac.25.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Tokuda H., Mizushima S. Proton motive force-dependent and -independent protein translocation revealed by an efficient in vitro assay system of Escherichia coli. J Biol Chem. 1989 Jan 25;264(3):1723–1728. [PubMed] [Google Scholar]
- Yang C. C., Konisky J. Colicin V-treated Escherichia coli does not generate membrane potential. J Bacteriol. 1984 May;158(2):757–759. doi: 10.1128/jb.158.2.757-759.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]


