Abstract
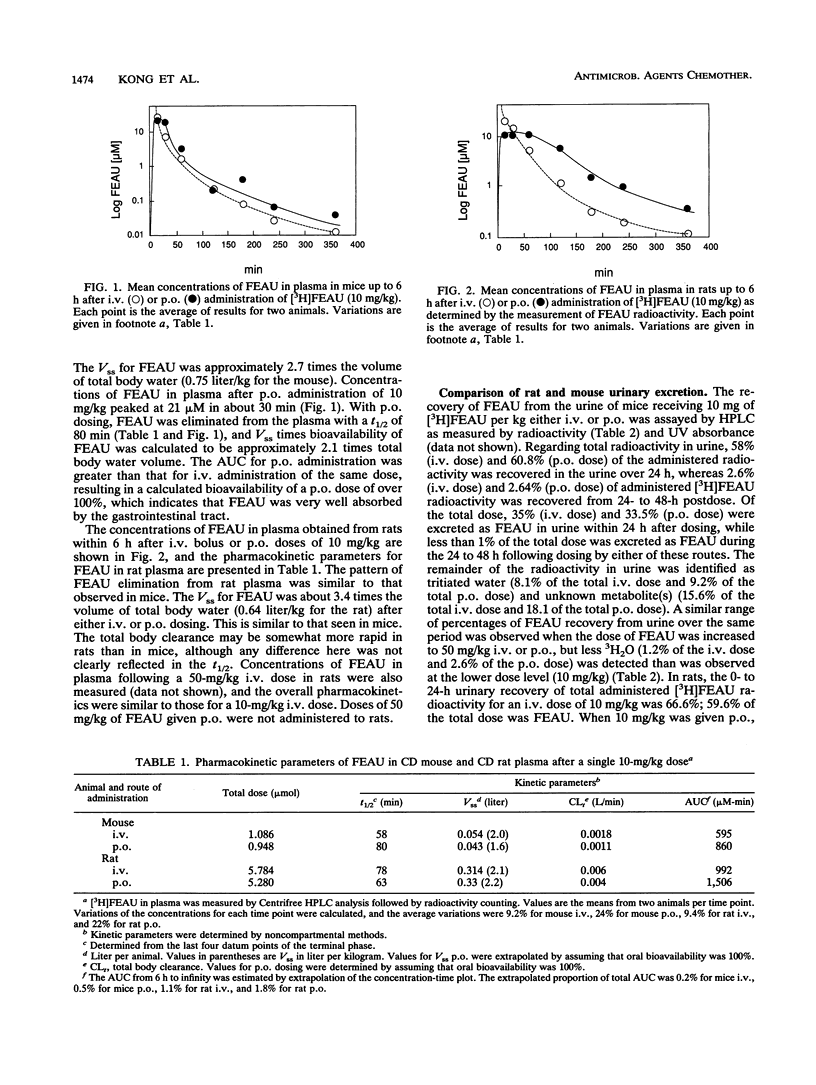
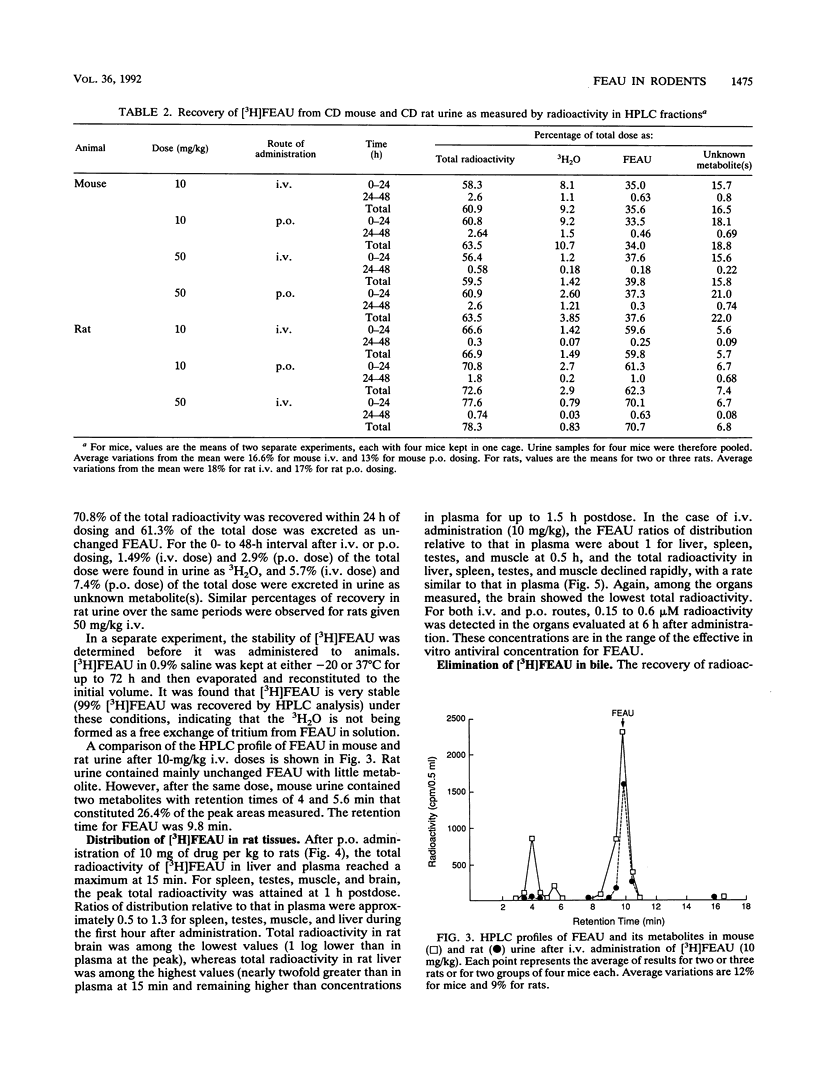
The preclinical pharmacology and pharmacokinetics of 2'-fluoro-5-ethyl-1-beta-D-arabinofuranosyluracil (FEAU), a selective inhibitor of herpesvirus and hepatitis virus replication, were investigated in the mouse and rat. Following intravenous (i.v.) or oral (p.o.) administration, FEAU was cleared from the plasma primarily unchanged, with a terminal half-life of 58 to 80 min in the mouse and 63 to 78 min in the rat. The steady-state volumes of distribution times bioavailabilities of FEAU were approximately 2.1 and 3.4 times the total body water volumes after p.o. administration of 10 mg of drug per kg of body weight in mice and rats, respectively. A comparison of the area under the concentration-time curve after i.v. and p.o. FEAU administration indicated that the p.o. dose was completely absorbed in both species. When tritiated FEAU was used in mice, 35.0% of the i.v. dose and 33.5% of the p.o. dose were excreted in urine as unchanged FEAU, 8.1% (i.v. dose) and 9.2% (p.o. dose) were excreted as tritiated water, and 15.6% (i.v. dose) and 18.1% (p.o. dose) were excreted as unknown metabolite(s) in urine within 24 h of dosing. Only 1.24% (i.v. dose) and 2.6% (p.o. dose) of the total doses were found in urine as 3H2O when the FEAU dose was increased to 50 mg/kg. However, a higher percentage of the total dose (59.6% for the i.v. dose and 61.3% for the p.o. dose) was recovered within 24 h as intact FEAU in rat urine, less than 1.4% (i.v. dose) and 2.7% (p.o. dose) of the total dose were found to be 3H2O, and 5.6% (i.v. dose) and 6.7% (p.o. dose) of the total dose were excreted as known metabolite(s). The distribution ratios for total radioactivity in tissue relative to those in plasma were 0.5 to 1.3 in spleen, testes, muscle, and liver during the first hour after a 10-mg/kg dose in rats. Of the total FEAU radioactivity administered, only 1.38% was excreted in bile as unchanged FEAU. No FEAU glucuronide metabolite was detected. Tissue concentrations of 0.15 to 0.6 microM at 6 h after dosing are in the range of the effective antiviral concentration for FEAU. In conclusion, FEAU administered p.o. to mice and rats was well absorbed; FEAU was rapidly distributed into tissues and remained above in vitro antiviral concentrations for more than 6 h; in mice, [3H]FEAU showed metabolism-mediated tritium exchange with water; and in rats, FEAU was less extensively metabolized than in mice and clearance was primarily via renal processes, mainly in the form of unchanged FEAU.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou T. C., Kong X. B., Fanucchi M. P., Cheng Y. C., Takahashi K., Watanabe K. A., Fox J. J. Synthesis and biological effects of 2'-fluoro-5-ethyl-1-beta-D-arabinofuranosyluracil. Antimicrob Agents Chemother. 1987 Sep;31(9):1355–1358. doi: 10.1128/aac.31.9.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlowski L. E., Jain R. K. Physiologically based pharmacokinetic modeling: principles and applications. J Pharm Sci. 1983 Oct;72(10):1103–1127. doi: 10.1002/jps.2600721003. [DOI] [PubMed] [Google Scholar]
- Kong X. B., Scheck A. C., Price R. W., Vidal P. M., Fanucchi M. P., Watanabe K. A., Fox J. J., Chou T. C. Incorporation and metabolism of 2'-fluoro-5-substituted arabinosyl pyrimidines and their selective inhibition of viral DNA synthesis in herpes simplex virus type 1 (HSV-1)-infected and mock-infected Vero cells. Antiviral Res. 1988 Dec 1;10(4-5):153–166. doi: 10.1016/0166-3542(88)90028-9. [DOI] [PubMed] [Google Scholar]
- Philips F. S., Feinberg A., Chou T. C., Vidal P. M., Su T. L., Watanabe K. A., Fox J. J. Distribution, metabolism, and excretion of 1-(2-fluoro-2-deoxy-beta-D- arabinofuranosyl)thymine and 1-(2-fluoro-2-deoxy-beta-D-arabinofuranosyl)-5- iodocytosine. Cancer Res. 1983 Aug;43(8):3619–3627. [PubMed] [Google Scholar]
- Soike K. F., Chou T. C., Fox J. J., Watanabe K. A., Gloff C. A. Inhibition of simian varicella virus infection of monkeys by 1-(2-deoxy-2- fluoro-1-beta-D-arabinofuranosyl)-5-ethyluracil (FEAU) and synergistic effects of combination with human recombinant interferon-beta. Antiviral Res. 1990 Apr;13(4):165–174. doi: 10.1016/0166-3542(90)90035-6. [DOI] [PubMed] [Google Scholar]
- Watanabe K. A., Matsuda A., Halat M. J., Hollenberg D. H., Nisselbaum J. S., Fox J. J. Nucleosides. 114. 5'-O-Glucuronides of 5-fluorouridine and 5-fluorocytidine. Masked precursors of anticancer nucleosides. J Med Chem. 1981 Jul;24(7):893–897. doi: 10.1021/jm00139a026. [DOI] [PubMed] [Google Scholar]
- Watanabe K. A., Reichman U., Hirota K., Lopez C., Fox J. J. Nucleosides. 110. Synthesis and antiherpes virus activity of some 2'-fluoro-2'-deoxyarabinofuranosylpyrimidine nucleosides. J Med Chem. 1979 Jan;22(1):21–24. doi: 10.1021/jm00187a005. [DOI] [PubMed] [Google Scholar]


