Abstract
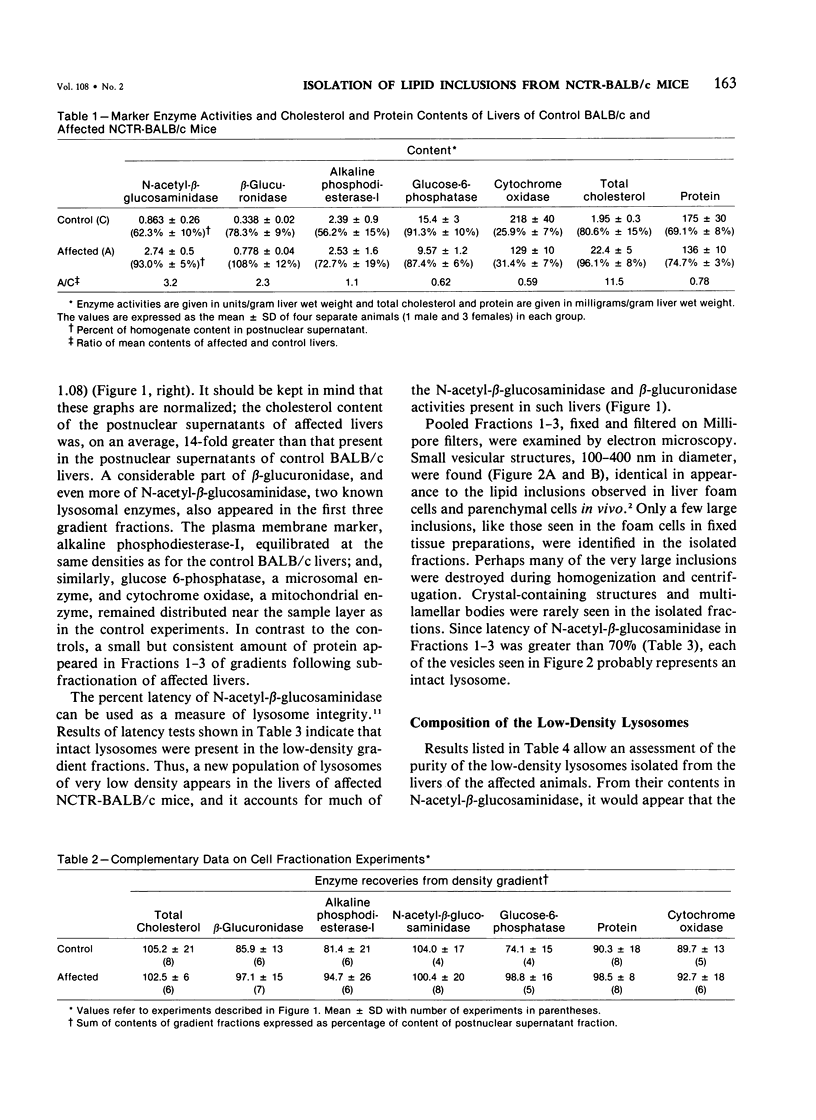
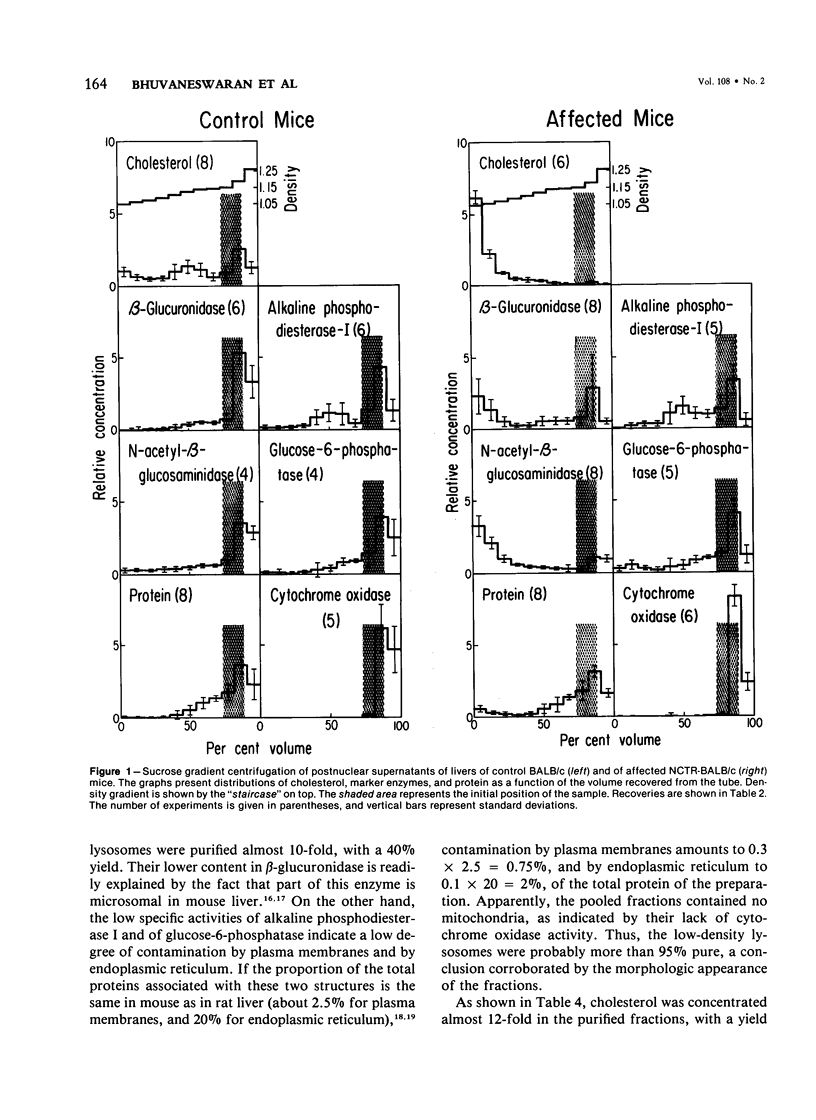
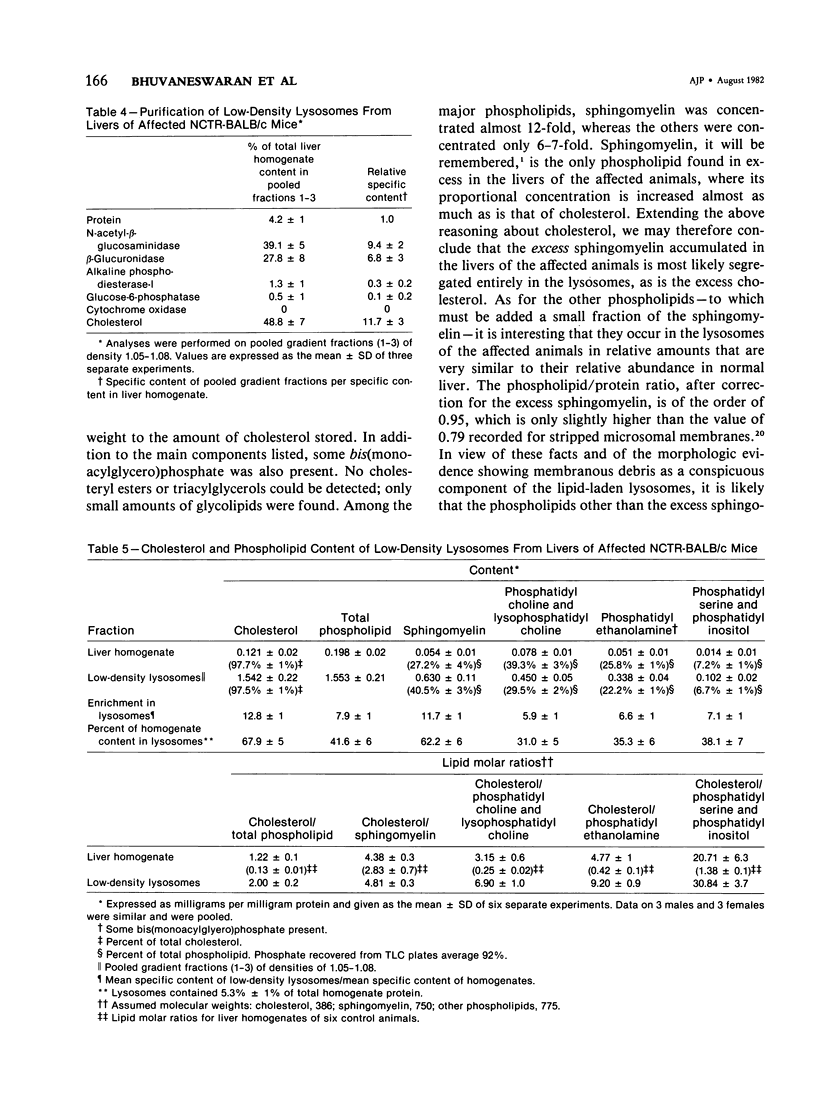
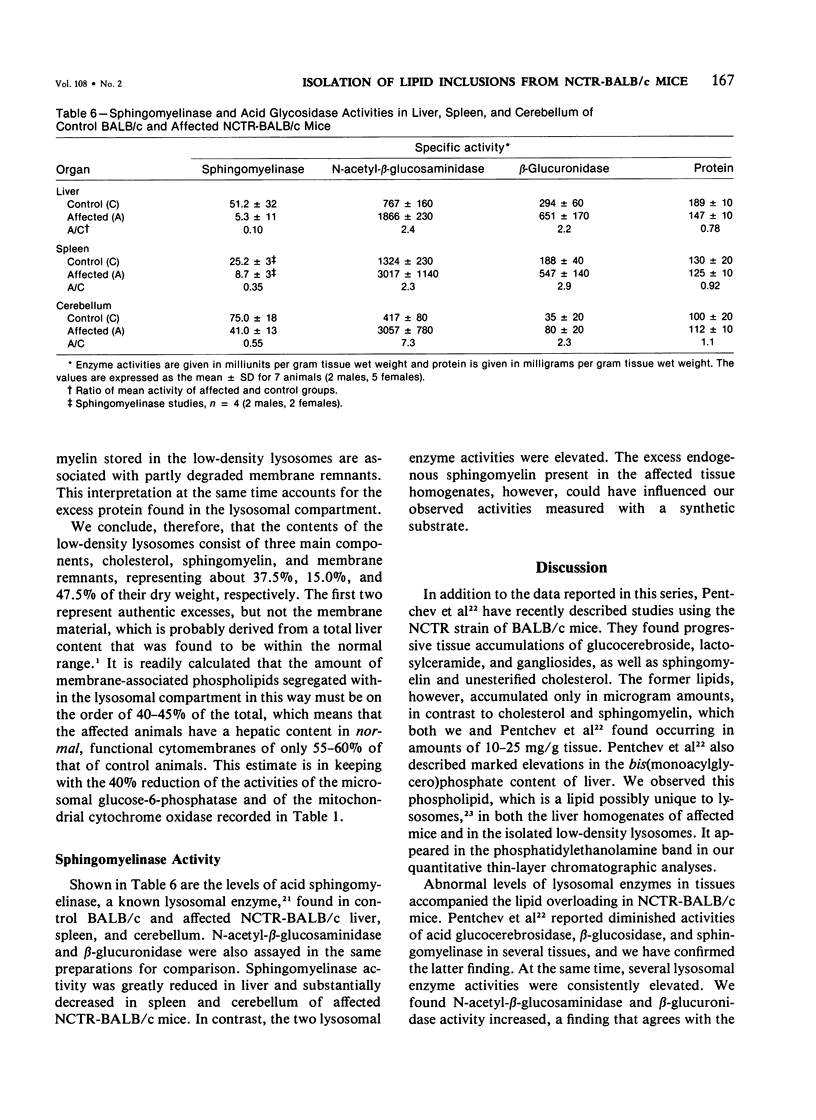
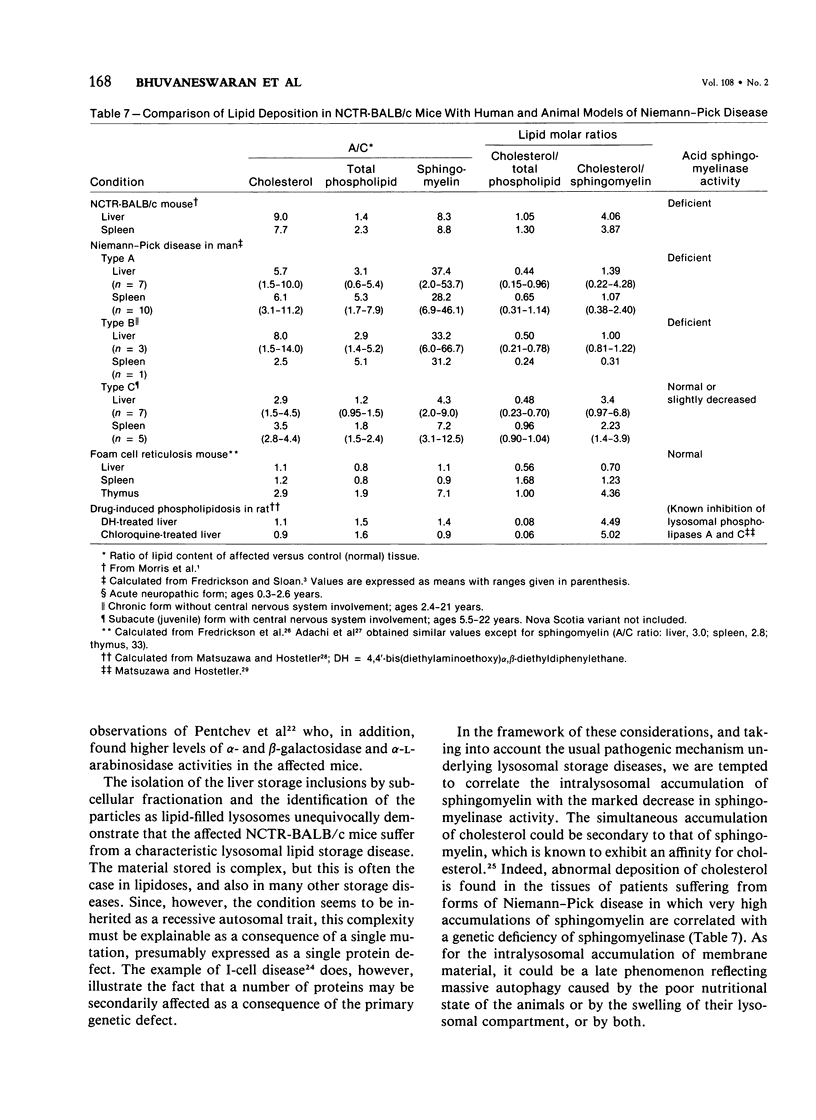
Livers of NCTR-BALB/c mice, affected by excessive accumulation of cholesterol and phospholipid, were fractionated by sucrose density gradient centrifugation. Lysosomes of very low density (rho = 1.05 - 1.08) were found, which by electron microscopy appeared identical to the storage inclusions seen in fixed tissues. These lysosomes could be purified about 10-fold over the original homogenate, and represented 4% of the total protein and 30-40% of the liver acid hydrolase content. The preparations were nearly free of mitochondrial, endoplasmic reticulum, and plasma membrane contamination. The lysosomes were laden with cholesterol and phospholipid. Cholesterol (greater than 97% unesterified) accounted for half of the total lipid, and sphingomyelin accounted for another 20%. Phosphatidylcholine and phosphatidylethanolamine were also present in substantial quantities. All of the excess cholesterol and sphingomyelin of liver could be attributed to the low density lysosomes. Lysosomal acid sphingomyelinase activity, measured with a synthetic substrate, was found to be 10-60% of BALB/c mouse control levels in liver, spleen, and cerebellum, while two other lysosomal enzymes, N-acetyl-beta-glucosaminidase and beta-glucuronidase, were increased 2-8-fold in the same tissues. These data and the morphologic observations of the preceding paper establish that the disorder affecting NCTR-BALB/c mice is a lysosome storage disease. We propose several possible mechanisms to explain the cholesterol and phospholipid overloading of lysosomes. The specific gene defect remains to be established.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi M., Tsai C. Y., Hoffman L. M., Schneck L., Volk B. W. The central nervous system, liver, and spleen of FM mice. Ultrastructural, histochemical, and biochemical studies. Arch Pathol. 1974 Apr;97(4):232–238. [PubMed] [Google Scholar]
- Adelman M. R., Sabatini D. D., Blobel G. Ribosome-membrane interaction. Nondestructive disassembly of rat liver rough microsomes into ribosomal and membranous components. J Cell Biol. 1973 Jan;56(1):206–229. doi: 10.1083/jcb.56.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudhuin P., Evrard P., Berthet J. Electron microscopic examination of subcellular fractions. I. The preparation of representative samples from suspensions of particles. J Cell Biol. 1967 Jan;32(1):181–191. doi: 10.1083/jcb.32.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufay H., Amar-Costesec A., Feytmans E., Thinès-Sempoux D., Wibo M., Robbi M., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J Cell Biol. 1974 Apr;61(1):188–200. doi: 10.1083/jcb.61.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. M., Poznansky M. J. Semipermeable microcapsules containing catalase for enzyme replacement in acatalasaemic mice. Nature. 1968 Apr 20;218(5138):243–245. doi: 10.1038/218243a0. [DOI] [PubMed] [Google Scholar]
- Coakley W. T., James C. J. A simple linear transform for the Folin-Lowry protein calibration curve to 1.0 mg/ml. Anal Biochem. 1978 Mar;85(1):90–97. doi: 10.1016/0003-2697(78)90278-6. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., WATTIAUX R. Tissue fractionation studies. VII. Release of bound hydrolases by means of triton X-100. Biochem J. 1956 Aug;63(4):606–608. doi: 10.1042/bj0630606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M. F., Muir H., Benson P., Button L. Enzyme replacement therapy in the mucopolysaccharidoses by fibroblast transplantation. Birth Defects Orig Artic Ser. 1980;16(1):445–456. [PubMed] [Google Scholar]
- Demel R. A., Jansen J. W., van Dijck P. W., van Deenen L. L. The preferential interaction of cholesterol with different classes of phospholipids. Biochim Biophys Acta. 1977 Feb 14;465(1):1–10. doi: 10.1016/0005-2736(77)90350-9. [DOI] [PubMed] [Google Scholar]
- Desnick R. J., Thorpe S. R., Fiddler M. B. Toward enzyme therapy for lysosomal storage diseases. Physiol Rev. 1976 Jan;56(1):57–99. doi: 10.1152/physrev.1976.56.1.57. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. Cell junctions in amphibian skin. J Cell Biol. 1965 Jul;26(1):263–291. doi: 10.1083/jcb.26.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S. Lysosomal localization of sphingomyelinase in rat liver. Biochim Biophys Acta. 1969 Nov 4;191(2):481–484. doi: 10.1016/0005-2744(69)90271-x. [DOI] [PubMed] [Google Scholar]
- Fowler S., Remacle J., Trouet A., Beaufay H., Berthet J., Wibo M., Hauser P. Analytical study of microsomes and isolated subcellular membranes from rat liver. V. Immunological localization of cytochrome b5 by electron microscopy: methodology and application to various subcellular fractions. J Cell Biol. 1976 Nov;71(2):535–550. doi: 10.1083/jcb.71.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson D. S., Sloan H. R., Hansen C. T. Lipid abnormalities in foam cell reticulosis of mice, an analogue of human sphingomyelin lipidosis. J Lipid Res. 1969 May;10(3):288–293. [PubMed] [Google Scholar]
- Gal A. E., Brady R. O., Hibbert S. R., Pentchev P. G. A practical chromogenic procedure for the detection of homozygotes and heterozygous carriers of Niemann-Pick disease. N Engl J Med. 1975 Sep 25;293(13):632–636. doi: 10.1056/NEJM197509252931304. [DOI] [PubMed] [Google Scholar]
- Gibbs D. A., Spellacy E., Roberts A. E., Watts R. W. The treatment of lysosomal storage diseases by fibroblast transplantation: some preliminary observations. Birth Defects Orig Artic Ser. 1980;16(1):457–474. [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa Y., Hostetler K. Y. Inhibition of lysosomal phospholipase A and phospholipase C by chloroquine and 4,4'-bis(diethylaminoethoxy) alpha, beta-diethyldiphenylethane. J Biol Chem. 1980 Jun 10;255(11):5190–5194. [PubMed] [Google Scholar]
- Matsuzawa Y., Hostetler K. Y. Studies on drug-induced lipidosis: subcellular localization of phospholipid and cholesterol in the liver of rats treated with chloroquine or 4,4'-bis (diethylaminoethoxy)alpha, beta-diethyldiphenylethane. J Lipid Res. 1980 Feb;21(2):202–214. [PubMed] [Google Scholar]
- Maziere J. C., Wolf C., Maziere C., Mora L., Bereziat G., Polonovski J. Inhibition of human fibroblasts sphingomyelinase by cholesterol and 7-dehydrocholesterol. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1299–1304. doi: 10.1016/0006-291x(81)91965-3. [DOI] [PubMed] [Google Scholar]
- Morris M. D., Bhuvaneswaran C., Shio H., Fowler S. Lysosome lipid storage disorder in NCTR-BALB/c mice. I. Description of the disease and genetics. Am J Pathol. 1982 Aug;108(2):140–149. [PMC free article] [PubMed] [Google Scholar]
- PAIGEN K. The effect of mutation on the intracellular location of beta-glucuronidase. Exp Cell Res. 1961 Nov;25:286–301. doi: 10.1016/0014-4827(61)90280-4. [DOI] [PubMed] [Google Scholar]
- Pentchev P. G., Gal A. E., Booth A. D., Omodeo-Sale F., Fouks J., Neumeyer B. A., Quirk J. M., Dawson G., Brady R. O. A lysosomal storage disorder in mice characterized by a dual deficiency of sphingomyelinase and glucocerebrosidase. Biochim Biophys Acta. 1980 Sep 8;619(3):669–679. doi: 10.1016/0005-2760(80)90116-2. [DOI] [PubMed] [Google Scholar]
- Remacle J., Fowler S., Beaufay H., Amarcostesec A., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. VI. Electron microscope examination of microsomes for cytochrome b5 by means of a ferritin-labeled antibody. J Cell Biol. 1976 Nov;71(2):551–564. doi: 10.1083/jcb.71.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallen T. J., Srikantaiah M. V., Seetharam B., Hansbury E., Gavey K. L. Proceedings: Sterol carrier protein hypothesis. Fed Proc. 1974 Jun;33(6):1733–1746. [PubMed] [Google Scholar]
- Shio H., Fowler S., Bhuvaneswaran C., Morris M. D. Lysosome lipid storage disorder in NCTR-BALB/c mice. II. Morphologic and cytochemical studies. Am J Pathol. 1982 Aug;108(2):150–159. [PMC free article] [PubMed] [Google Scholar]
- Shio H., Haley N. J., Fowler S. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. III. Intracellular localization of cholesterol and cholesteryl ester. Lab Invest. 1979 Aug;41(2):160–167. [PubMed] [Google Scholar]
- Slavin S., Yatziv S. Correction of enzyme deficiency in mice by allogeneic bone marrow transplantation with total lymphoid irradiation. Science. 1980 Dec 5;210(4474):1150–1152. doi: 10.1126/science.7003711. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J., Erecińska M., Chance B. Cytochrome c interaction with membranes. II. Comparative study of the interaction of c cytochromes with the mitochondrial membrane. Arch Biochem Biophys. 1973 Aug;157(2):531–540. doi: 10.1016/0003-9861(73)90672-3. [DOI] [PubMed] [Google Scholar]
- WALKER P. G. The preparation and properties of beta-glucuronidase. III. Fractionation and activity of homogenates in isotonic media. Biochem J. 1952 May;51(2):223–232. doi: 10.1042/bj0510223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Bloomgarden D., Kaplan R., Cohen C., Hoffstein S., Collins T., Gotlieb A., Nagle D. A general method for the introduction of enzymes, by means of immunoglobulin-coated liposomes, into lysosomes of deficient cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):88–92. doi: 10.1073/pnas.72.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherrett J. R., Huterer S. Enrichment of bis-(monoacylglyceryl) phosphate in lysosomes from rat liver. J Biol Chem. 1972 Jul 10;247(13):4114–4120. [PubMed] [Google Scholar]




