Abstract
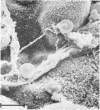
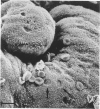
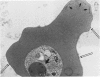
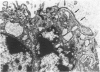
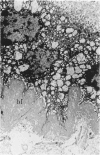
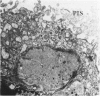
Placenta malarial changes (PMCs) related to maternal plasmodium infection were present in 33% (247 cases) of a series of 741 placentas collected from an unselected population living in an area of high malarial endemicity (Haut-Ogooué, Gabon, Africa). Plasmodia were found on material thick blood films taken at the time of delivery in 42% of the women with and 24% of women without associated PMCs. Plasmodium falciparum was the most frequent infecting organism. PMCs were more frequent and, in general, more marked in primiparas. The primiparas were significantly (P less than 0.001) more numerous in the group with PMCs than in the control group without such changes. The mean weight of term placentas with malarial changes was significantly (46 g; P less than 0.001) less than that of placentas without such changes. The morphologic changes were a combination of the following features: 1) presence of parasites in the intervillous spaces; 2) macrophage concentration in the intervillous spaces; 3) malarial pigment deposits; 4) excess of perivillous fibrinoid deposits; 5) syncytiotrophoblastic damage; and 6) trophoblastic basal lamina thickening. Plasmodia were found in placental intervillous spaces in 42% (105/247). Local parasitemia varied in magnitude; in a few cases, 30% or more of the maternal erythrocytes were infected. Macrophage concentration in the intervillous spaces was present in 29% (72/247) and was always associated with local parasitemia. Macrophages phagocytized red blood cells and malarial pigment, and their number varied inversely with that of the local parasites. It seems, therefore, that macrophages play an important role in local parasite clearance. Malarial brown pigment was observed in all cases from the series. It had characteristic ultrastructural features and occurred in perivillous deposits of fibrinoid, in macrophages, or free in intervillous spaces. Excessive perivillous fibrinoid deposits were a constant histologic finding and were usually associated with syncytiotrophoblastic necrosis or ultrastructural damage such as partial microvilli loss, filamentous material accumulation in intracytoplasmic vacuoles, and "podocytelike" cytoplasmic projections on the basal surface. At these sites the trophoblastic basal lamina was usually thickened. Previously reported morphologic data and our own findings suggest that the peculiar placental changes in malaria, restricted to intervillous spaces and to villous surfaces, may be related to an immunopathologic process.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHIBALD H. M. The influence of malarial infection of the placenta on the incidence of prematurity. Bull World Health Organ. 1956;15(3-5):842–845. [PMC free article] [PubMed] [Google Scholar]
- BRUCE-CHWATT L. J. Malaria in African infants and children in Southern Nigeria. Ann Trop Med Parasitol. 1952 Sep;46(2):173–200. doi: 10.1080/00034983.1952.11685522. [DOI] [PubMed] [Google Scholar]
- Bray R. S., Sinden R. E. The sequestration of Plasmodium falciparum infected erythrocytes in the placenta. Trans R Soc Trop Med Hyg. 1979;73(6):716–719. doi: 10.1016/0035-9203(79)90028-2. [DOI] [PubMed] [Google Scholar]
- CANNON D. S. Malaria and prematurity in the western region of Nigeria. Br Med J. 1958 Oct 11;2(5101):877–878. doi: 10.1136/bmj.2.5101.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. M., Bruce A., Rencricca N. J. Malaria: macrophage migration inhibition factor (MIF). J Parasitol. 1976 Feb;62(1):137–138. [PubMed] [Google Scholar]
- Crane G. G. The pathogenesis of tropical splenomegaly syndrome--the role of immune complexes. P N G Med J. 1977 Mar;20(1):6–13. [PubMed] [Google Scholar]
- Galbraith R. M., Fox H., Hsi B., Galbraith G. M., Bray R. S., Faulk W. P. The human materno-foetal relationship in malaria. II. Histological, ultrastructural and immunopathological studies of the placenta. Trans R Soc Trop Med Hyg. 1980;74(1):61–72. doi: 10.1016/0035-9203(80)90012-7. [DOI] [PubMed] [Google Scholar]
- Hindi R. D., Azimi P. H. Congenital malaria due to Plasmodium falciparum. Pediatrics. 1980 Dec;66(6):977–979. [PubMed] [Google Scholar]
- Kilejian A., Abati A., Trager W. Plasmodium falciparum and Plasmodium coatneyi: immunogenicity of "knob-like protrusions" on infected erythrocyte membranes. Exp Parasitol. 1977 Jun;42(1):157–164. doi: 10.1016/0014-4894(77)90073-x. [DOI] [PubMed] [Google Scholar]
- Logie D. E., McGregor I. A. Acute malaria in newborn infants. Br Med J. 1970 Aug 15;3(5719):404–405. doi: 10.1136/bmj.3.5719.404-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neva F. A., Howard W. A., Glew R. H., Krotoski W. A., Gam A. A., Collins W. E., Atkinson J. P., Frank M. M. Relationship of serum complement levels to events of the malarial paroxysm. J Clin Invest. 1974 Aug;54(2):451–460. doi: 10.1172/JCI107781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin L. H., Mackey L., Aguado T., Lambert P. H., Miescher P. A. Démonstration d'antigènes parasitaires solubles, d'anticorps correspondants et de complexes immuns lors de malaria aiguë. Schweiz Med Wochenschr. 1979 Dec 1;109(46):1832–1834. [PubMed] [Google Scholar]
- Rosenberg E. B., Strickland G. T., Yang S. L., Whalen G. E. IgM antibodies to red cells and autoimmune anemia in patients with malaria. Am J Trop Med Hyg. 1973 Mar;22(2):146–152. doi: 10.4269/ajtmh.1973.22.146. [DOI] [PubMed] [Google Scholar]
- SPITZ A. J. Malaria infection of the placenta and its influence on the incidence of prematurity in eastern Nigeria. Bull World Health Organ. 1959;21:242–244. [PMC free article] [PubMed] [Google Scholar]
- Scheibel L. W., Ashton S. H., Trager W. Plasmodium falciparum: microaerophilic requirements in human red blood cells. Exp Parasitol. 1979 Jun;47(3):410–418. doi: 10.1016/0014-4894(79)90094-8. [DOI] [PubMed] [Google Scholar]
- Thomas V., Chit C. W. A case of congenital malaria in Malaysia with IgM malaria antibodies. Trans R Soc Trop Med Hyg. 1980;74(1):73–76. doi: 10.1016/0035-9203(80)90014-0. [DOI] [PubMed] [Google Scholar]
- Toro G., Román G. Cerebral malaria. A disseminated vasculomyelinopathy. Arch Neurol. 1978 May;35(5):271–275. doi: 10.1001/archneur.1978.00500290017004. [DOI] [PubMed] [Google Scholar]
- Tubbs H. Endotoxin in human and murine malaria. Trans R Soc Trop Med Hyg. 1980;74(1):121–123. doi: 10.1016/0035-9203(80)90026-7. [DOI] [PubMed] [Google Scholar]
- Vincent H. M., Wilson R. J. Malarial antigens on infected erythrocytes. Trans R Soc Trop Med Hyg. 1980;74(4):452–455. doi: 10.1016/0035-9203(80)90052-8. [DOI] [PubMed] [Google Scholar]
- Walter P., Garin J. F., Blot P., Philippe E. Placenta et paludisme. Etude morphologique, parasitologique et clinique. J Gynecol Obstet Biol Reprod (Paris) 1981;10(6):535–542. [PubMed] [Google Scholar]