Abstract
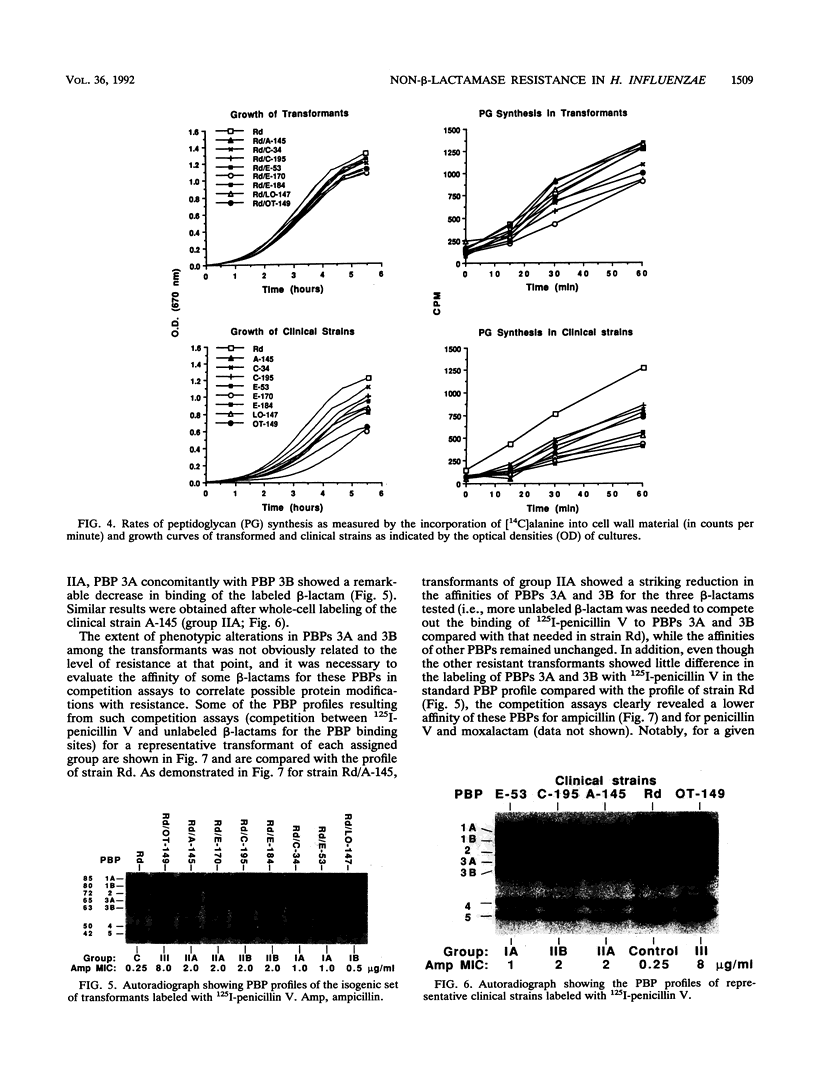
A study recently conducted across Canada showed that 64 of 2,503 clinical isolates of Haemophilus influenzae were resistant to beta-lactams without production of a beta-lactamase (L. D. Tremblay, J. L'Ecuyer, P. Provencher, M. G. Bergeron, and Canadian Study Group, Can. Med. Assoc. J. 143:895-900, 1990). The beta-lactamase-negative strains formed three distinct groups, with ampicillin MICs of 0.5 to 1, 2 to 4, and greater than or equal to 8 micrograms/ml for groups I, II, and III, respectively. We have investigated the mechanisms of resistance for eight strains originating from different infections and geographic areas. These strains were representative of groups I to III. Five strains were nontypeable, two were type B, and one was non-B. Chromosomal DNA extracted from each strain was used to transform the laboratory strain Rd. Transformants were selected on beta-lactam-containing plates and showed the same level of resistance to ampicillin as the donor strains. Differences in outer membrane proteins, porins, and lipopolysaccharide profiles on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) did not change with resistance. Functional analyses of purified porins in artificial lipid bilayer experiments did not explain resistance. Peptidoglycan synthesis was measured by incorporation of [14C]alanine into trichloroacetic acid-insoluble cell wall material in the presence of chloramphenicol. The growth rate and the rate of peptidoglycan synthesis observed for the transformants of the isogenic set did not correlate with resistance. Whole-cell labeling with 125I-penicillin revealed modifications in penicillin-binding proteins (PBPs) among the transformants. In particular, PBPs 3A and 3B (65 and 63 kDa, respectively) showed a decrease in affinity for beta-lactams in all transformants (groups I, II, and III) and correlated with an increased MIC except in the transformant of group III, which showed higher levels of resistance. Partial purification and proteolytic digestion of 125I-penicillin-labeled PBP 3B led to two types of CnBr peptide profiles on SDS-PAGE, the profiles of the transformed strains from groups I and II being different from those of the control group and group III. Finally, electron microscopy revealed a distinct cell filamentation for the group III transformants. These data clearly indicate that changes in PBPs are a common mechanism that results in a significant level of non-beta-lactamase-mediated beta-lactam resistance in H. influenzae despite serotype, origin of isolation, or geographic distribution.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. E., Hobbs J. N., Alborn W. E., Jr Inhibition of peptidoglycan biosynthesis in gram-positive bacteria by LY146032. Antimicrob Agents Chemother. 1987 Jul;31(7):1093–1099. doi: 10.1128/aac.31.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp D., Pellerin M., Gourde P., Pettigrew M., Bergeron M. G. Effects of daptomycin and vancomycin on tobramycin nephrotoxicity in rats. Antimicrob Agents Chemother. 1990 Jan;34(1):139–147. doi: 10.1128/aac.34.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlone G. M., Thomas M. L., Rumschlag H. S., Sottnek F. O. Rapid microprocedure for isolating detergent-insoluble outer membrane proteins from Haemophilus species. J Clin Microbiol. 1986 Sep;24(3):330–332. doi: 10.1128/jcm.24.3.330-332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S., Malouin F., Rabin H. R., Schollaardt T., Parr T. R., Jr, Bryan L. E. Persistence of Pseudomonas aeruginosa during ciprofloxacin therapy of a cystic fibrosis patient: transient resistance to quinolones and protein F-deficiency. J Antimicrob Chemother. 1990 Jun;25(6):995–1010. doi: 10.1093/jac/25.6.995. [DOI] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., Dorrance D. The permeability barrier of Haemophilus influenzae type b against beta-lactam antibiotics. J Antimicrob Chemother. 1983 Nov;12(5):435–449. doi: 10.1093/jac/12.5.435. [DOI] [PubMed] [Google Scholar]
- Handwerger S., Tomasz A. Antibiotic tolerance among clinical isolates of bacteria. Rev Infect Dis. 1985 May-Jun;7(3):368–386. doi: 10.1093/clinids/7.3.368. [DOI] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lugtenberg E. J., de Haan P. G. A simple method for following the fate of alanine-containing components in murein synthesis in Escherichia coli. Antonie Van Leeuwenhoek. 1971;37(4):537–552. doi: 10.1007/BF02218524. [DOI] [PubMed] [Google Scholar]
- Malouin F., Bryan L. E. Modification of penicillin-binding proteins as mechanisms of beta-lactam resistance. Antimicrob Agents Chemother. 1986 Jul;30(1):1–5. doi: 10.1128/aac.30.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouin F., Bryan L. E., Shewciw P., Douglas J., Li D., Van den Elzen H., Lapointe J. R. DNA probe technology for rapid detection of Haemophilus influenzae in clinical specimens. J Clin Microbiol. 1988 Oct;26(10):2132–2138. doi: 10.1128/jcm.26.10.2132-2138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouin F., Campbell G. D., Halpenny M., Becker G. W., Parr T. R., Jr Outer membrane and porin characteristics of Serratia marcescens grown in vitro and in rat intraperitoneal diffusion chambers. Infect Immun. 1990 May;58(5):1247–1253. doi: 10.1128/iai.58.5.1247-1253.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouin F., Chamberland S., Brochu N., Parr T. R., Jr Influence of growth media on Escherichia coli cell composition and ceftazidime susceptibility. Antimicrob Agents Chemother. 1991 Mar;35(3):477–483. doi: 10.1128/aac.35.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouin F., Parr T. R., Jr, Bryan L. E. Identification of a group of Haemophilus influenzae penicillin-binding proteins that may have complementary physiological roles. Antimicrob Agents Chemother. 1990 Feb;34(2):363–365. doi: 10.1128/aac.34.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouin F., Schryvers A. B., Bryan L. E. Cloning and expression of genes responsible for altered penicillin-binding proteins 3a and 3b in Haemophilus influenzae. Antimicrob Agents Chemother. 1987 Feb;31(2):286–291. doi: 10.1128/aac.31.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelman P. M., Chaffin D. O., Krilov L. R., Kalaitzoglou G., Serfass D. A., Onay O., Wiley E. A., Overturf G. D., Rubin L. G. Cefuroxime treatment failure of nontypable Haemophilus influenzae meningitis associated with alteration of penicillin-binding proteins. J Infect Dis. 1990 Nov;162(5):1118–1123. doi: 10.1093/infdis/162.5.1118. [DOI] [PubMed] [Google Scholar]
- Mendelman P. M., Chaffin D. O., Stull T. L., Rubens C. E., Mack K. D., Smith A. L. Characterization of non-beta-lactamase-mediated ampicillin resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1984 Aug;26(2):235–244. doi: 10.1128/aac.26.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Apicella M. A. Nontypable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune response to infection. Rev Infect Dis. 1987 Jan-Feb;9(1):1–15. doi: 10.1093/clinids/9.1.1. [DOI] [PubMed] [Google Scholar]
- Parr T. R., Jr, Bryan L. E. Mechanism of resistance of an ampicillin-resistant, beta-lactamase-negative clinical isolate of Haemophilus influenzae type b to beta-lactam antibiotics. Antimicrob Agents Chemother. 1984 Jun;25(6):747–753. doi: 10.1128/aac.25.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston D. A., Wu C. Y., Blaszczak L. C., Seitz D. E., Halligan N. G. Biological characterization of a new radioactive labeling reagent for bacterial penicillin-binding proteins. Antimicrob Agents Chemother. 1990 May;34(5):718–721. doi: 10.1128/aac.34.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfass D. A., Mendelman P. M., Chaffin D. O., Needham C. A. Ampicillin resistance and penicillin-binding proteins of Haemophilus influenzae. J Gen Microbiol. 1986 Oct;132(10):2855–2861. doi: 10.1099/00221287-132-10-2855. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature. 1988 Mar 10;332(6160):173–176. doi: 10.1038/332173a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Stewart G. J., Carlson C. A. The biology of natural transformation. Annu Rev Microbiol. 1986;40:211–235. doi: 10.1146/annurev.mi.40.100186.001235. [DOI] [PubMed] [Google Scholar]
- Tremblay L. D., L'Ecuyer J., Provencher P., Bergeron M. G. Susceptibility of Haemophilus influenzae to antimicrobial agents used in Canada. Canadian Study Group. CMAJ. 1990 Nov 1;143(9):895–901. [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Durack D. T., Tomasz A. Antibiotic tolerance among clinical isolates of bacteria. Antimicrob Agents Chemother. 1986 Oct;30(4):521–527. doi: 10.1128/aac.30.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon V., Kristjanson D. N., Coulton J. W. Outer membrane porin protein of Haemophilus influenzae type b: pore size and subunit structure. Can J Microbiol. 1988 Feb;34(2):134–140. doi: 10.1139/m88-027. [DOI] [PubMed] [Google Scholar]
- Vachon V., Laprade R., Coulton J. W. Properties of the porin of Haemophilus influenzae type b in planar lipid bilayer membranes. Biochim Biophys Acta. 1986 Sep 25;861(1):74–82. doi: 10.1016/0005-2736(86)90373-1. [DOI] [PubMed] [Google Scholar]
- Vachon V., Lyew D. J., Coulton J. W. Transmembrane permeability channels across the outer membrane of Haemophilus influenzae type b. J Bacteriol. 1985 Jun;162(3):918–924. doi: 10.1128/jb.162.3.918-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum R. R., Amanuma H., O'Brien T. A., Waxman D. J., Strominger J. L. Penicillin is an active-site inhibitor for four genera of bacteria. J Bacteriol. 1982 Mar;149(3):1150–1153. doi: 10.1128/jb.149.3.1150-1153.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zighelboim S., Tomasz A. Penicillin-binding proteins of multiply antibiotic-resistant South African strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):434–442. doi: 10.1128/aac.17.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]