Abstract
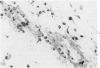
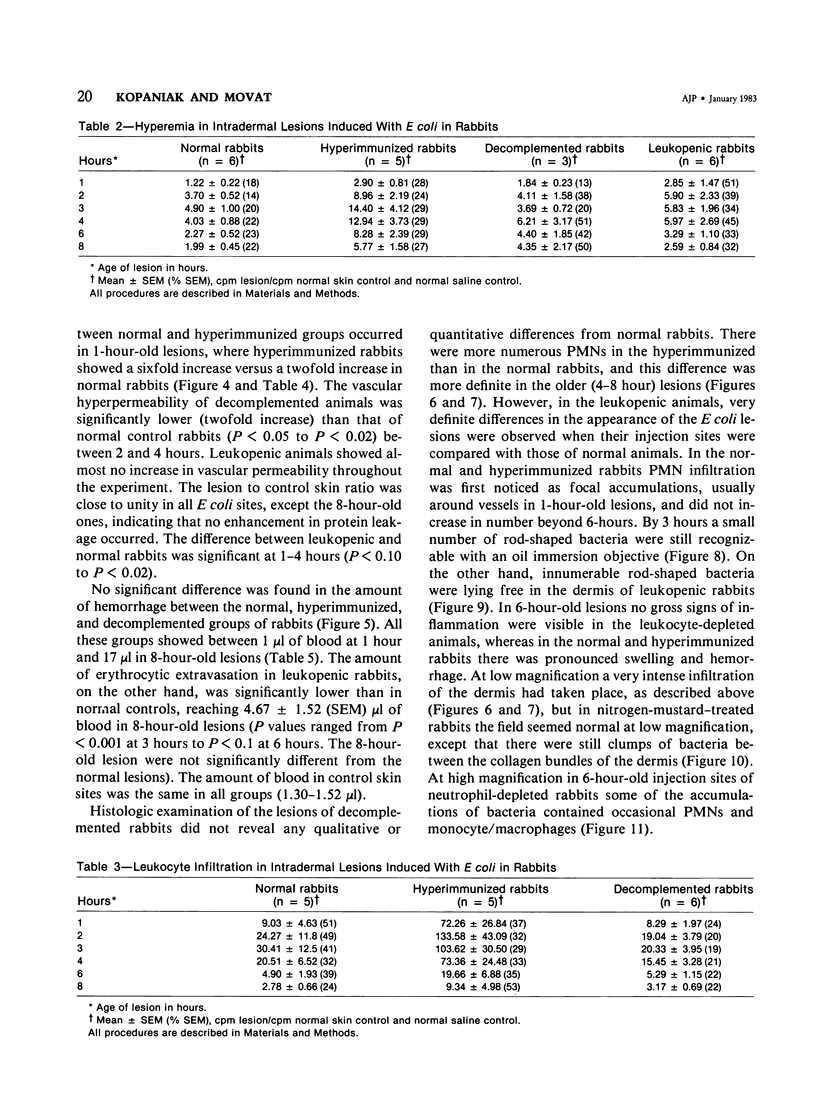
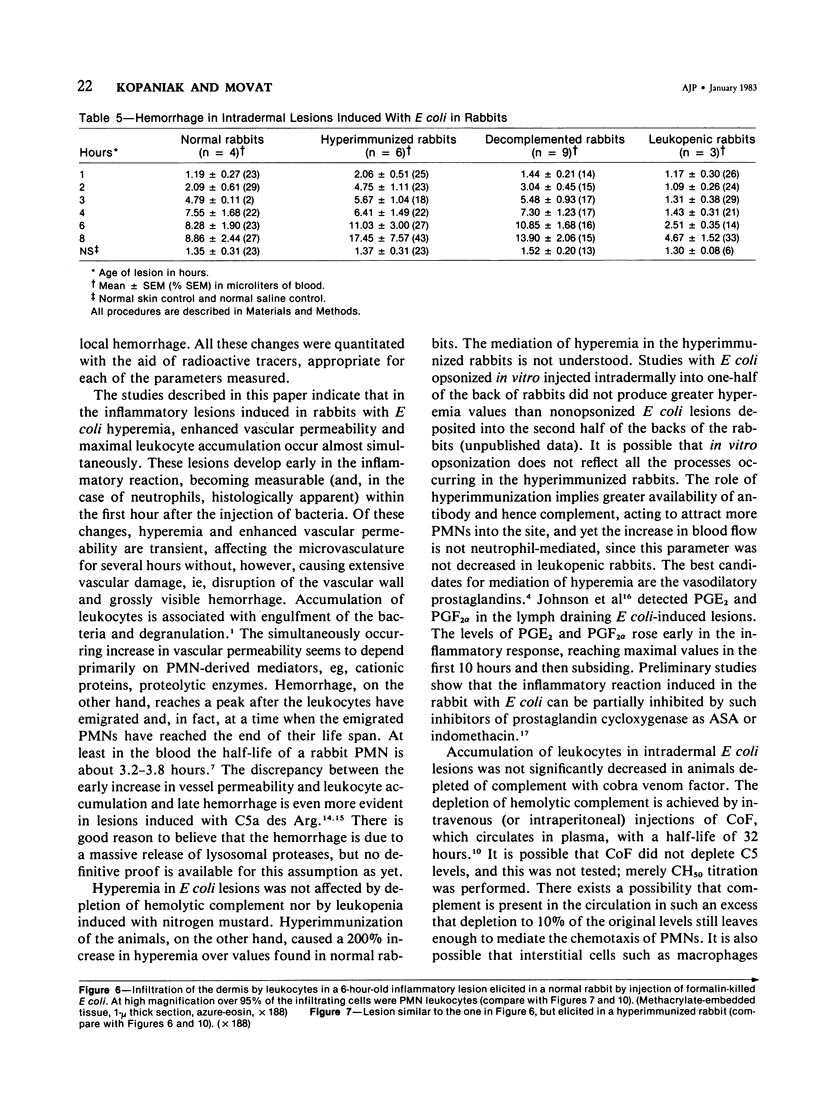
The inflammatory response to Escherichia coli was quantitated in the skin of normal rabbits and the kinetics established as described previously. Hyperemia, measured with radiolabeled microspheres; vascular permeability, estimated with 125 I-albumin; and leukocyte infiltration, quantitated with 51Cr-labeled autologous leukocytes, reached maximal values 3 hours after the injection of bacteria and subsided almost completely by 6 hours. Hemorrhage, measured with homologous 59Fe-erythrocytes, continued to increase between 1 and 6 hours after injection and then reached plateau levels. The lesions were studied up to 8 hours, since in the previous study no changes were observed beyond that time. In the study described in this paper, the host mediation systems were manipulated in various groups of rabbits in order to elucidate the mechanisms underlying the development of the inflammatory reaction. One group of animals was hyperimmunized with the E coli organisms, another was partially depleted of hemolytic complement with cobra venom factor, and yet another was rendered leukopenic with nitrogen mustard. In hyperimmunized animals hyperemia in the dermal lesions induced by the microorganisms was significantly more intense than in normal rabbits. Vascular permeability increase occurred earlier in hyperimmunized rabbits and at 1 hour was significantly greater than in normals. Decomplemented rabbits had significantly less vascular permeability than normal animals, whereas in leukopenic rabbits no increase in vascular permeability could be elicited. Leukocyte accumulation was increased over the normal animals in the lesions of hyperimmunized rabbits. Hemorrhage was significantly decreased in leukopenic rabbits. Histologic examination of the lesions revealed that whereas in normal animals the infiltrating neutrophils ingested most of the bacteria and formed definite abscesses by 6-8 hours, these abscesses were absent in leukopenic animals, and free-lying bacteria were demonstrable in lesions. Histologically more neutrophils were present in the hyperimmunized than in the normal rabbits, but this difference was striking when normal animals were compared with leukopenic animals, in some of which only very occasional small accumulations of neutrophils were present.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chenoweth D. E., Hugli T. E. Human C5a and C5a analogs as probes of the neutrophil C5a receptor. Mol Immunol. 1980 Feb;17(2):151–161. doi: 10.1016/0161-5890(80)90067-x. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Gerard C., Chenoweth D. E., Hugli T. E. Molecular aspects of the serum chemotactic factors. J Reticuloendothel Soc. 1979 Dec;26(Suppl):711–718. [PubMed] [Google Scholar]
- HURLEY J. V. ACUTE INFLAMMATION: THE EFFECT OF CONCURRENT LEUCOCYTIC EMIGRATION AND INCREASED PERMEABILITY ON PARTICLE RETENTION BY THE VASCULAR WALL. Br J Exp Pathol. 1964 Dec;45:627–633. [PMC free article] [PubMed] [Google Scholar]
- HURLEY J. V., SPECTOR W. G. Delayed leucocytic emigration after intra-dermal injections and thermal injury. J Pathol Bacteriol. 1961 Oct;82:421–429. doi: 10.1002/path.1700820219. [DOI] [PubMed] [Google Scholar]
- HURLEY J. V. SUBSTANCES PROMOTING LEUKOCYTE EMIGRATION. Ann N Y Acad Sci. 1964 Aug 27;116:918–935. doi: 10.1111/j.1749-6632.1964.tb52558.x. [DOI] [PubMed] [Google Scholar]
- Hay J. B., Johnston M. G., Hobbs B. B., Movat H. Z. The use of radioactive microspheres to quantitate hyperemia in dermal inflammatory sites. Proc Soc Exp Biol Med. 1975 Dec;150(3):641–644. doi: 10.3181/00379727-150-39096. [DOI] [PubMed] [Google Scholar]
- Hugli T. E., Müller-Eberhard H. J. Anaphylatoxins: C3a and C5a. Adv Immunol. 1978;26:1–53. doi: 10.1016/s0065-2776(08)60228-x. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C., Bhimji S. The effect of nonsteroidal anti-inflammatory agents on E. coli-induced inflammation. Immunopharmacology. 1982 Feb;4(1):11–22. doi: 10.1016/0162-3109(82)90022-4. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C., Movat H. Z. The effect of vasodilator prostaglandins on polymorphonuclear leukocyte infiltration and vascular injury. Am J Pathol. 1982 Jun;107(3):300–309. [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Movat H. Z. The in vivo quantitation and kinetics of rabbit neutrophil leukocyte accumulation in the skin in response to chemotactic agents and Escherichia coli. Lab Invest. 1980 Mar;42(3):310–317. [PubMed] [Google Scholar]
- Issekutz A. C., Movat K. W., Movat H. Z. Enhanced vascular permeability and haemorrhage-inducing activity of rabbit C5ades arg: probable role of polymorphonuclear leucocyte lysosomes. Clin Exp Immunol. 1980 Sep;41(3):512–520. [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Movat K. W., Movat H. Z. Enhanced vascular permeability and haemorrhage-inducing activity of zymosan-activated plasma. Clin Exp Immunol. 1980 Sep;41(3):505–511. [PMC free article] [PubMed] [Google Scholar]
- Issekutz T. B., Issekutz A. C., Movat H. Z. The in vivo quantitation and kinetics of monocyte migration into acute inflammatory tissue. Am J Pathol. 1981 Apr;103(1):47–55. [PMC free article] [PubMed] [Google Scholar]
- Johnston M. G., Hay J. B., Movat H. Z. Kinetics of prostaglandin production in various inflammatory lesions, measured in draining lymph. Am J Pathol. 1979 Apr;95(1):225–238. [PMC free article] [PubMed] [Google Scholar]
- Kopaniak M. M., Hay J. B., Movat H. Z. The effect of hyperemia on vascular permeability. Microvasc Res. 1978 Jan;15(1):77–82. doi: 10.1016/0026-2862(78)90007-9. [DOI] [PubMed] [Google Scholar]
- Kopaniak M. M., Issekutz A. C., Burrowes C. E., Movat H. Z. The quantitation of hemorrhage in the skin. Measurement of hemorrhage in the microcirculation in inflammatory lesions and related phenomena. Proc Soc Exp Biol Med. 1980 Jan;163(1):126–131. doi: 10.3181/00379727-163-40733. [DOI] [PubMed] [Google Scholar]
- Kopaniak M. M., Issekutz A. C., Movat H. Z. Kinetics of acute inflammation induced by E coli in rabbits. Quantitation of blood flow, enhanced vascular permeability, hemorrhage, and leukocyte accumulation. Am J Pathol. 1980 Feb;98(2):485–498. [PMC free article] [PubMed] [Google Scholar]
- Larsen G. L., Mitchell B. C., Henson P. M. The pulmonary response of C5 sufficient and deficient mice to immune complexes. Am Rev Respir Dis. 1981 Apr;123(4 Pt 1):434–439. doi: 10.1164/arrd.1981.123.4.434. [DOI] [PubMed] [Google Scholar]
- Minta J. O., Man D. Immunological, structural and functional relationships between an anti-complementary protein from Crotalus atrox venom, cobra venom factor and human C3. Immunology. 1980 Apr;39(4):503–509. [PMC free article] [PubMed] [Google Scholar]
- Reyes M. P. The aerobic gram-negative bacillary pneumonias. Med Clin North Am. 1980 May;64(3):363–383. doi: 10.1016/s0025-7125(16)31598-x. [DOI] [PubMed] [Google Scholar]
- Sahu S., Lynn W. S. Lipid chemotaxins isolated from culture filtrates of Escherichia coli and from oxidized lipids. Inflammation. 1977 Mar;2(1):47–54. doi: 10.1007/BF00920874. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Showell H. V., Corcoran B. A., Ward P. A., Smith E., Becker E. L. The isolation and partial characterization of neutrophil chemotactic factors from Escherichia coli. J Immunol. 1975 Jun;114(6):1831–1837. [PubMed] [Google Scholar]
- THOMAS L., GOOD R. A. Studies on the generalized Shwartzman reaction: I. General observations concerning the phenomenon. J Exp Med. 1952 Dec;96(6):605–624. doi: 10.1084/jem.96.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tainer J. A., Turner S. R., Lynn W. S. New aspects of chemotaxis. Specific target-cell attraction by lipid and lipoprotein fractions of Escherichia coli chemotactic factor. Am J Pathol. 1975 Nov;81(2):401–410. [PMC free article] [PubMed] [Google Scholar]
- Udaka K., Takeuchi Y., Movat H. Z. Simple method for quantitation of enhanced vascular permeability. Proc Soc Exp Biol Med. 1970 Apr;133(4):1384–1387. doi: 10.3181/00379727-133-34695. [DOI] [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Wilson D. M., Ormrod D. J., Miller T. E. Role of complement in chemotaxis: study of a localized infection. Infect Immun. 1980 Jul;29(1):8–12. doi: 10.1128/iai.29.1.8-12.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]