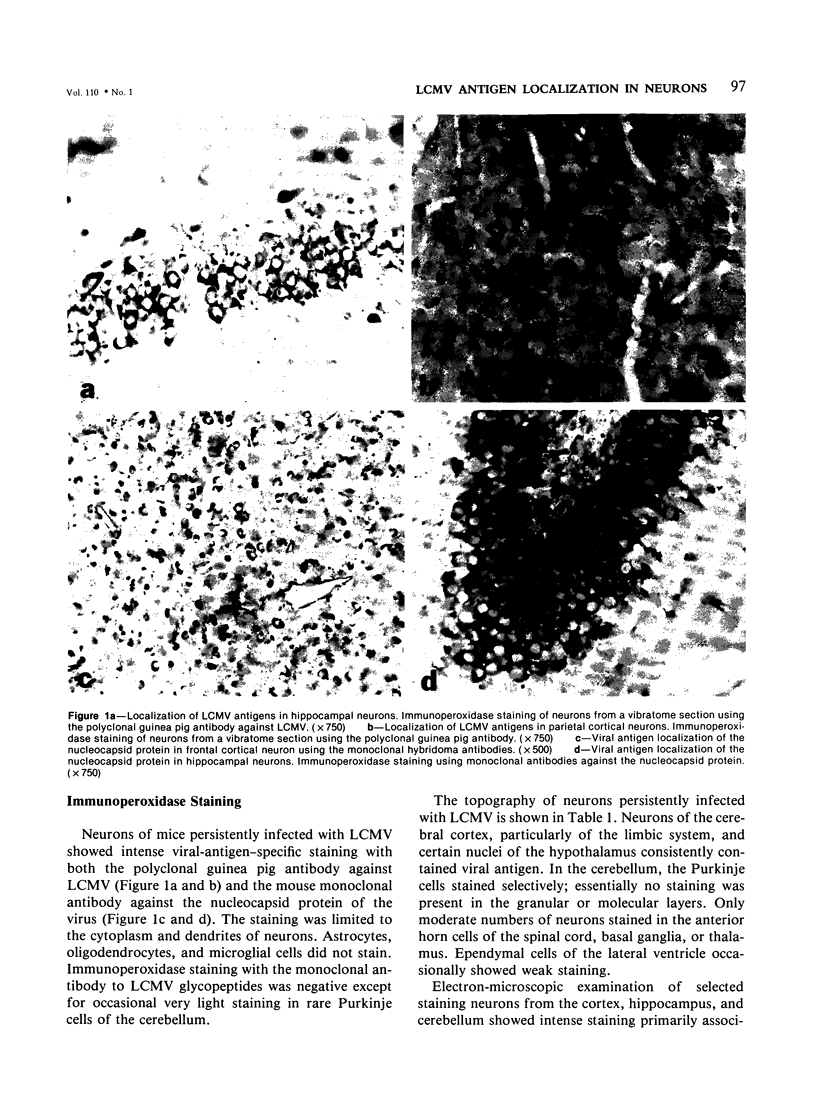
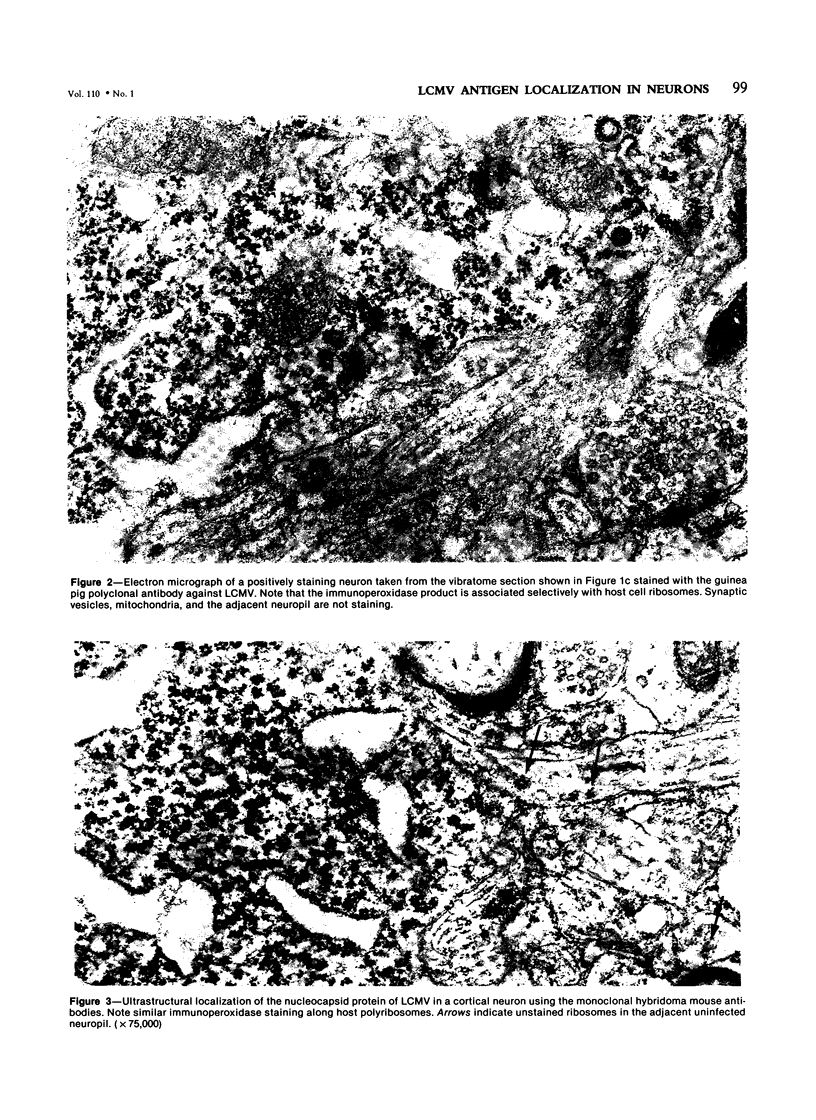
Abstract
Lymphocytic choriomeningitis virus (LCMV) produces a persistent infection of the nervous system in susceptible mice. To map the localization of viral antigens in the central nervous system (CNS), the authors studied, by means of ultrastructural immune peroxidase techniques, 4-6-month-old mice persistently infected with LCMV following an intracerebral inoculation at birth. The greatest number of infected neurons was observed in the cortex, particularly of the limbic system, and certain nuclei of the hypothalamus. In the cerebellum, Purkinje cells selectively expressed viral antigens. Moderate numbers of infected neurons were found in the anterior horns of the spinal cord, basal ganglia, and thalamus. The immunoperoxidase technique using monoclonal antibodies showed that persistently infected neurons primarily expressed the nucleocapsid protein antigens of LCMV. Glycopeptide antigens were minimally expressed. Electron-microscopic examination of selected individual infected neurons showed viral antigens exclusively associated with ribosomes. No staining was seen on cell surfaces. Glutaraldehyde-fixed CNS tissue studied by electron microscopy did not reveal morphologic abnormalities or mature viral particles. This study demonstrates that LCMV persistently infects specific neuronal populations. Infected neurons express viral antigens in association with host ribosomes and show no significant morphologic alterations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Smith G. H., Hoffman H. A., Rowe W. P. Use of enzyme-labeled antibody for electron microscope localization of lymphocytic choriomeningitis virus antigens in infected cell cultures. J Natl Cancer Inst. 1969 Mar;42(3):497–515. [PubMed] [Google Scholar]
- Buchmeier M. J., Lewicki H. A., Tomori O., Oldstone M. B. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology. 1981 Aug;113(1):73–85. doi: 10.1016/0042-6822(81)90137-9. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Oldstone M. B. Virus-induced immune complex disease: identification of specific viral antigens and antibodies deposited in complexes during chronic lymphocytic choriomeningitis virus infection. J Immunol. 1978 Apr;120(4):1297–1304. [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- HOTCHIN J. The biology of lymphocytic choriomeningitis infection: virus-induced immune disease. Cold Spring Harb Symp Quant Biol. 1962;27:479–499. doi: 10.1101/sqb.1962.027.001.046. [DOI] [PubMed] [Google Scholar]
- Hotchin J., Seegal R. Virus-induced behavioral alteration of mice. Science. 1977 May 6;196(4290):671–674. doi: 10.1126/science.854742. [DOI] [PubMed] [Google Scholar]
- Mims C. A. Immunofluorescence study of the carrier state and mechanism of vertical transmission in lymphocytic choriomeningitis virus infection in mice. J Pathol Bacteriol. 1966 Apr;91(2):395–402. doi: 10.1002/path.1700910214. [DOI] [PubMed] [Google Scholar]
- Mims C. A. Observations on mice infected congenitally or neonatally with lymphocytic choriomeningitis (LCM) virus. Arch Gesamte Virusforsch. 1970;30(1):67–74. doi: 10.1007/BF01262584. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Aging and chronic virus infection: is there a relationship? Fed Proc. 1974 Sep;33(9):2057–2059. [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Holmstoen J., Welsh R. M., Jr Alterations of acetylcholine enzymes in neuroblastoma cells persistently infected with lymphocytic choriomeningitis virus. J Cell Physiol. 1977 Jun;91(3):459–472. doi: 10.1002/jcp.1040910316. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Murphy F. A., Whitfield S. G., Bauer S. P. Lymphocytic choriomeningitis: ultrastructural pathology. Exp Mol Pathol. 1975 Oct;23(2):245–265. doi: 10.1016/0014-4800(75)90022-2. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Buchmeier M. J. Protein analysis of defective interfering lymphocytic choriomeningitis virus and persistently infected cells. Virology. 1979 Jul 30;96(2):503–515. doi: 10.1016/0042-6822(79)90107-7. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Oldstone M. B. Inhibition of immunologic injury of cultured cells infected with lymphocytic choriomeningitis virus: role of defective interfering virus in regulating viral antigenic expression. J Exp Med. 1977 Jun 1;145(6):1449–1468. doi: 10.1084/jem.145.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]





