Abstract
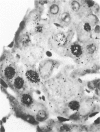
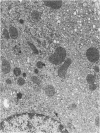
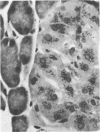
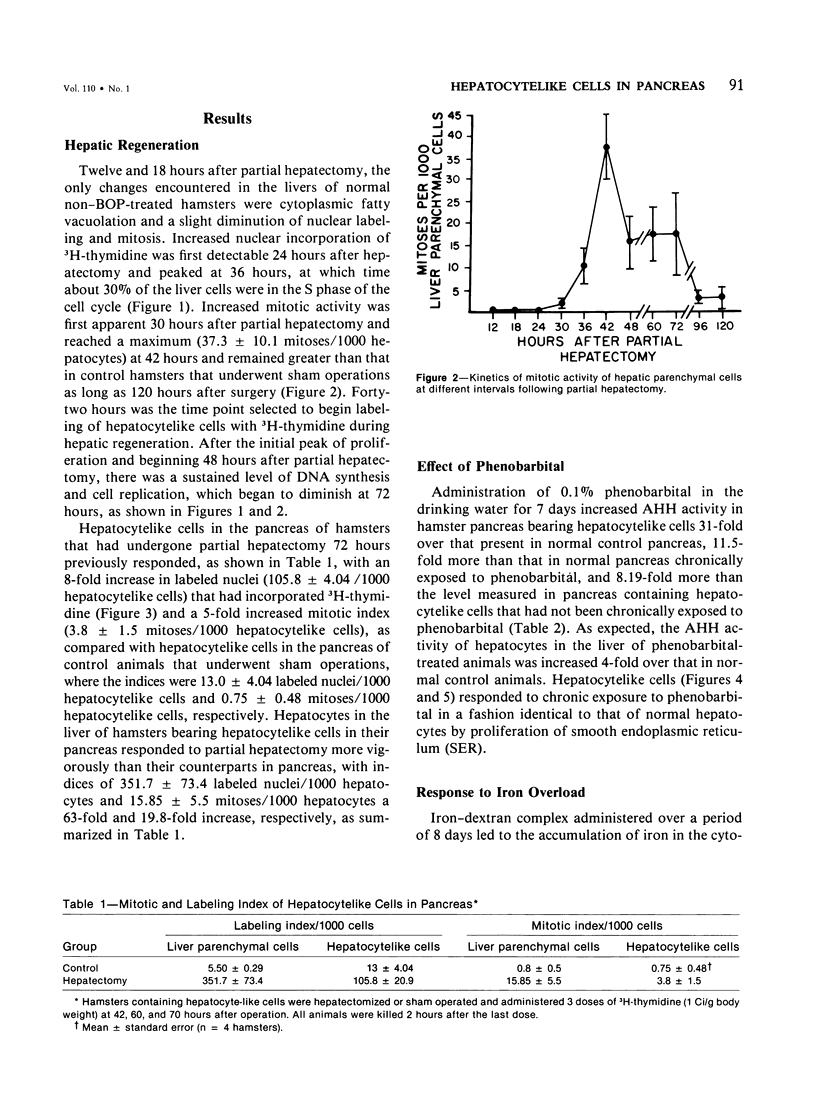
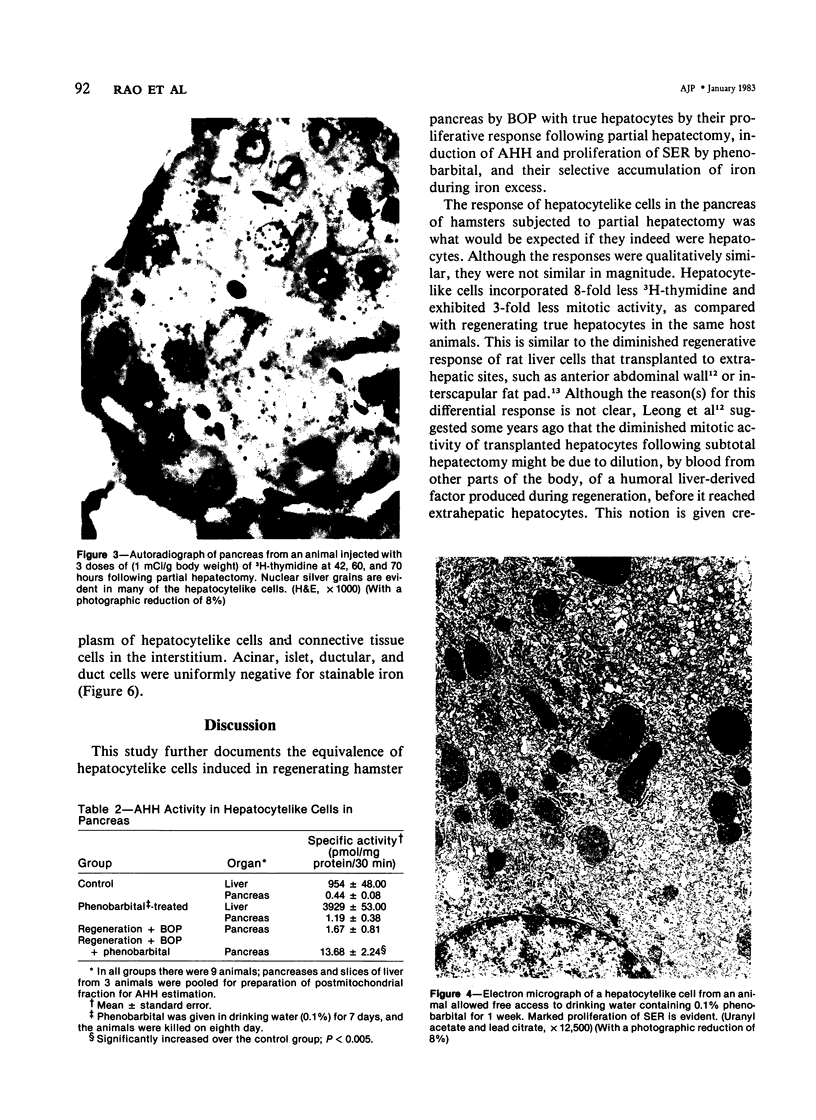
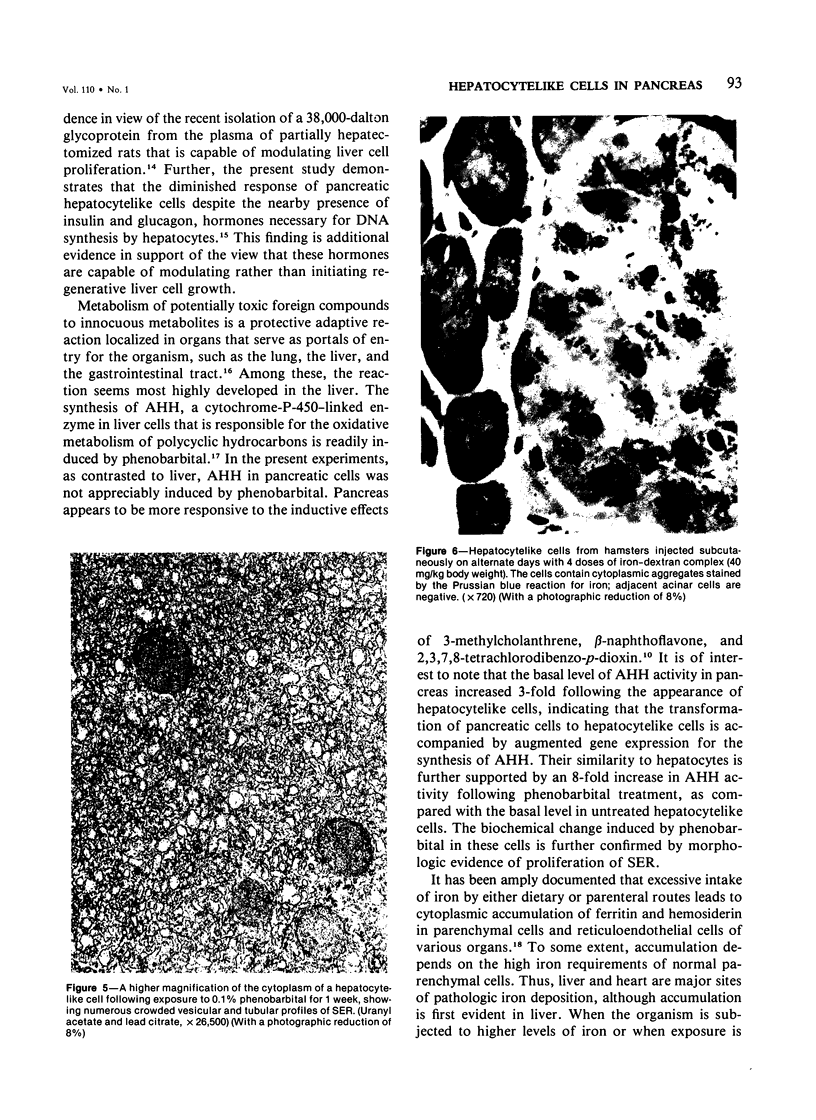
Regenerating pancreatic cells of the Syrian hamster treated at the peak of S phase with the pancreatic carcinogen N-nitrosobis(2-oxopropyl)amine (BOP) are converted into stable cells wtih morphologic and functional characteristics that are strikingly similar to those of differentiated hepatocytes. In this article the authors further document their hepatocytelike nature. Seventy-two hours after subtotal hepatectomy, pancreatic hepatocytelike cells responded with an 8-fold increase in labeled nuclei (105.8 +/- 4.04/1000 cells) which had incorporated 3H-thymidine and a 5-fold increased mitotic index (3.8 +/- 1.5 mitoses/1000 cells), as compared with similar cells in the pancreas of control animals that had undergone sham operations. Chronic administration of phenobarbital induced a 31-fold increase in the level of aryl hydrocarbon hydroxylase (AHH) in pancreas containing such cells, as compared with normal control pancreases, and caused marked proliferation of smooth endoplasmic reticulum (SER). These cells also showed an enhanced capacity for the accumulation of iron during acute iron excess, as compared with adjacent acinar cells. Collectively, these findings support the view that carcinogen-induced cells in pancreas bear a close functional resemblance to hepatocytes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bucher M. L., Swaffield M. N. Regulation of hepatic regeneration in rats by synergistic action of insulin and glucagon. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1157–1160. doi: 10.1073/pnas.72.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernozemsky I. N., Nikolov I. G., Stoichev I. I. Partial hepatectomy and liver regeneration in Syrian hamsters. Lab Anim. 1978 Apr;12(2):61–62. doi: 10.1258/002367778780953242. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Goldberg M., Strecker W., Feeny D., Ruhenstroth-Bauer G. Evidence for and characterization of a liver cell proliferation factor from blood plasma of partially hepatectomized rats. Horm Metab Res. 1980 Mar;12(3):94–96. doi: 10.1055/s-2007-996212. [DOI] [PubMed] [Google Scholar]
- Jirtle R. L., Michalopoulos G. Effects of partial hepatectomy on transplanted hepatocytes. Cancer Res. 1982 Aug;42(8):3000–3004. [PubMed] [Google Scholar]
- LEONG G. F., GRISHAM J. W., HOLE B. V., ALBRIGHT M. L. EFFECT OF PARTIAL HEPATECTOMY ON DNA SYNTHESIS AND MITOSIS IN HETEROTOPIC PARTIAL AUTOGRAFTS OF RAT LIVER. Cancer Res. 1964 Sep;24:1496–1501. [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. I. Assay and properties of induced enzyme. J Biol Chem. 1968 Dec 10;243(23):6242–6249. [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. The in vivo and in vitro induction of aryl hydrocarbon hydroxylase in mammalian cells of different species, tissues, strains, and developmental and hormonal states. Arch Biochem Biophys. 1969 Oct;134(1):76–89. doi: 10.1016/0003-9861(69)90253-7. [DOI] [PubMed] [Google Scholar]
- Pechet G. S. Parenteral iron overload. Organ and cell distribution in rats. Lab Invest. 1969 Jan;20(1):119–126. [PubMed] [Google Scholar]
- Rao M. S., Reddy M. K., Reddy J. K., Scarpelli D. G. Response of chemically induced hepatocytelike cells in hamster pancreas to methyl clofenapate, a peroxisome proliferator. J Cell Biol. 1982 Oct;95(1):50–56. doi: 10.1083/jcb.95.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K., Rao M. S., Svoboda D. J., Prasad J. D. Pancreatic necrosis and regeneration induced by 4-hydroxyaminoquinoline-1-oxide in the guinea pig. Lab Invest. 1975 Jan;32(1):98–104. [PubMed] [Google Scholar]
- Rogers A. E., Anderson G. H., Lenhardt G. M., Wolf G., Newberne P. M. A semisynthetic diet for long-term maintenance of hamsters to study effects of dietary vitamin A. Lab Anim Sci. 1974 Jun;24(3):495–499. [PubMed] [Google Scholar]
- Scarpelli D. G., Rao M. S. Differentiation of regenerating pancreatic cells into hepatocyte-like cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2577–2581. doi: 10.1073/pnas.78.4.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpelli D. G., Rao M. S., Subbarao V., Beversluis M., Gurka D. P., Hollenberg P. F. Activation of nitrosamines to mutagens by postmitochondrial fraction of hamster pancreas. Cancer Res. 1980 Jan;40(1):67–74. [PubMed] [Google Scholar]
- Scarpelli D. G., Rao M. S., Subbarao V., Beversluis M. Regeneration of Syrian golden hamster pancreas and covalent binding of N-nitroso-2,6-[3H]dimethylmorpholine. Cancer Res. 1981 Mar;41(3):1051–1057. [PubMed] [Google Scholar]