Abstract
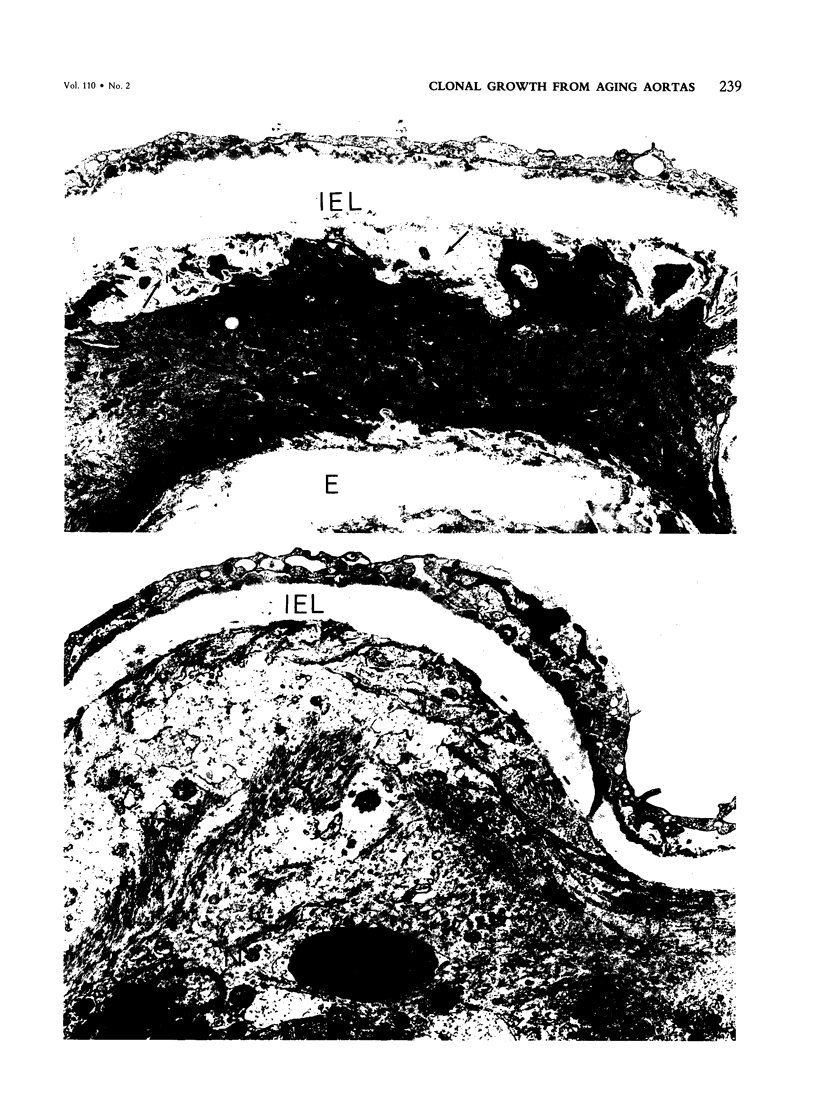
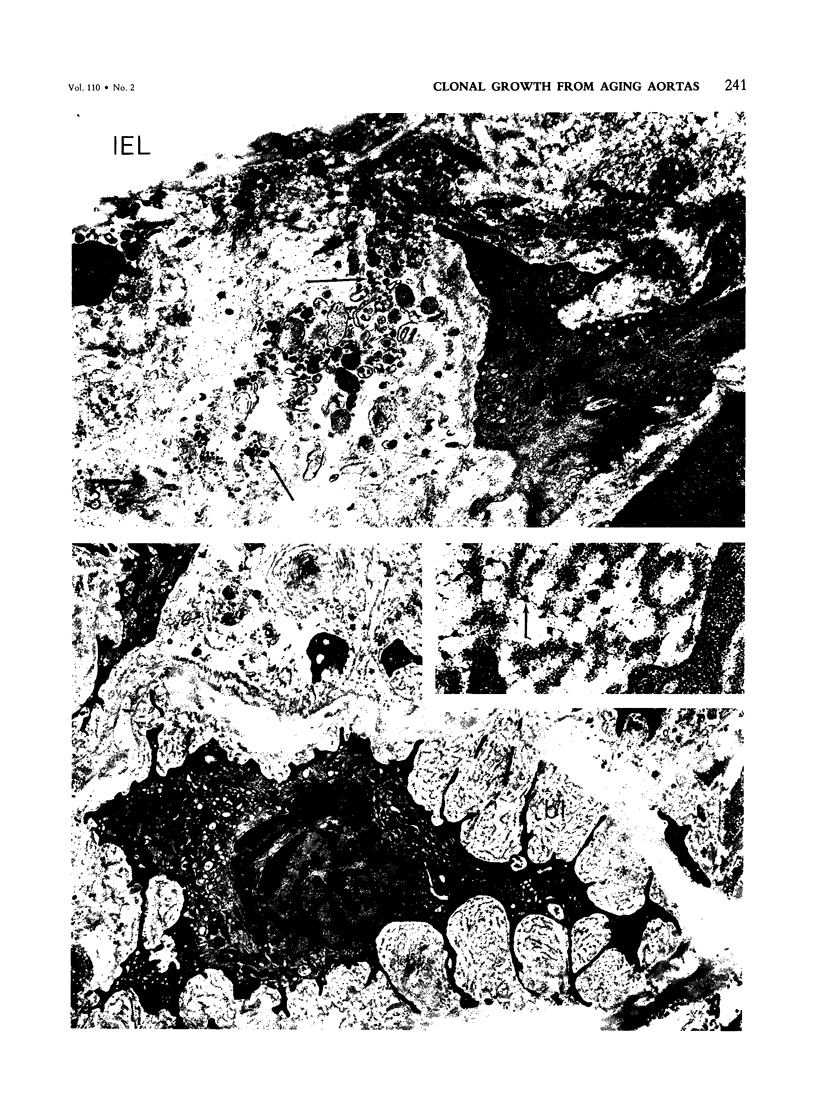
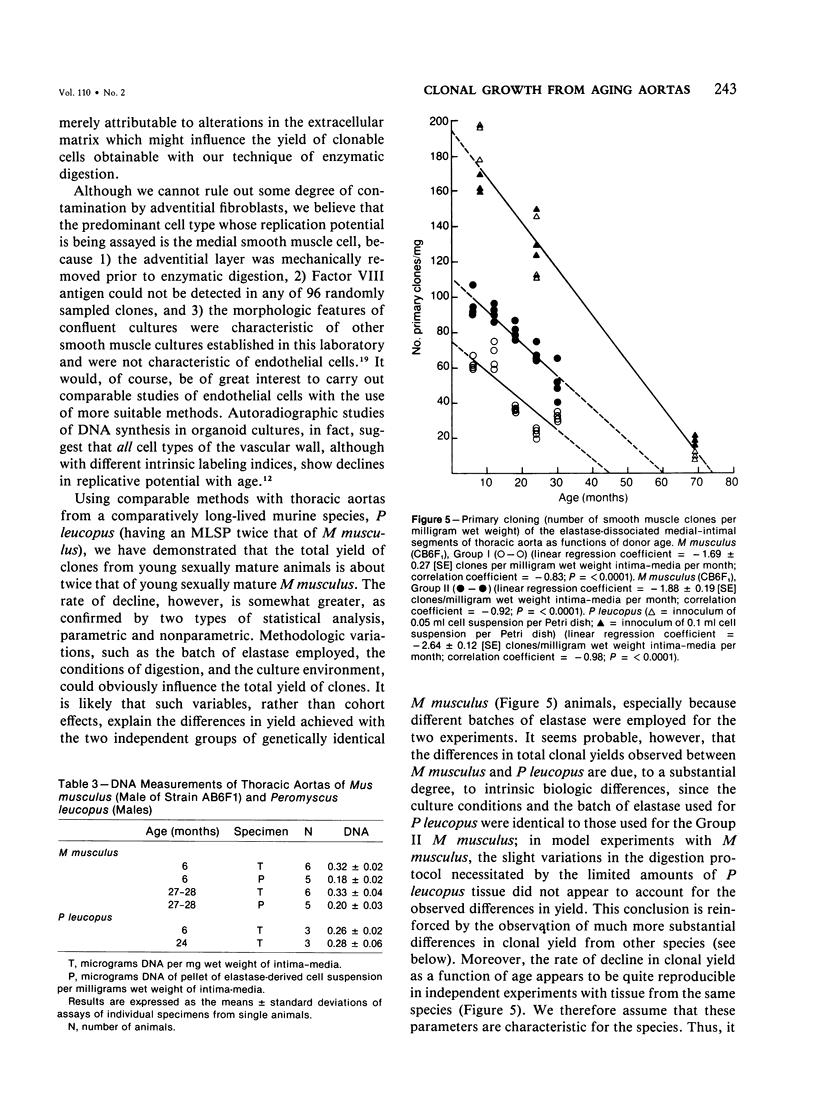
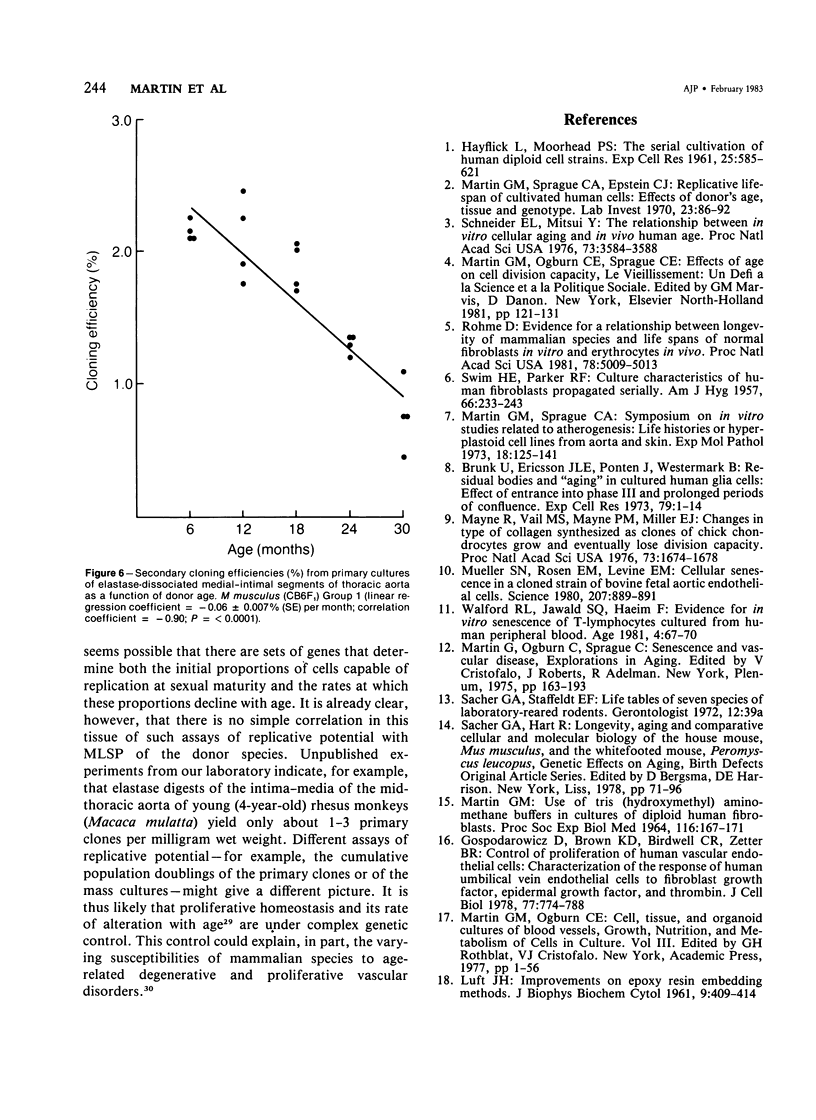
Primary cloning assays of thoracic aortic smooth muscle cells from an F1 hybrid strain of Mus musculus demonstrated linear regressions of replicative potentials as functions of donor age (6-30 months), with regression coefficients, in two independent cohorts, of -1.69 +/- 0.27 (SE) and -1.88 +/- 0.19 (SE) clones per milligram wet weight of intima-media per month and correlation coefficients of -0.83 and -0.92 (P less than 0.001). Secondary cloning (dilute plating from first passages) also demonstrated a high negative correlation (r = -0.90) between donor age and cloning efficiency, thus implicating intrinsic differences in cell populations. Comparable primary cloning assays on the aortas of aging cohorts of Peromyscus leucopus, a murine species with a maximum life span potential approximately twice that of Mus musculus, yielded about twice the number of clonable smooth muscle cells per unit weight; the rate of decline with age was slightly but significantly greater (P less than 0.01). Electron-microscopic studies revealed cellular alterations confined to the first subintimal layer of aortas from mice (Mus musculus) 18 months and older.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunk U., Ericsson J. L., Pontén J., Westermark B. Residual bodies and "aging" in cultured human glia cells. Effect of entrance into phase 3 and prolonged periods of confluence. Exp Eye Res. 1973 Apr;79(1):1–14. [PubMed] [Google Scholar]
- Cliff W. J. The aortic tunica media in aging rats. Exp Mol Pathol. 1970 Oct;13(2):172–189. doi: 10.1016/0014-4800(70)90004-3. [DOI] [PubMed] [Google Scholar]
- DEN TONKELAAREM, VAN DUIJN PHOTOGRAPHIC COLORIMETRY AS A QUANTITATIVE CYTOCHEMICAL METHOD. 3. DETERMINATION OF THE ABSOLUTE AMOUNT OF DNA IN CELL NUCLEI. Z Zellforch Microsk Anat Histochem. 1964;79:16–19. [PubMed] [Google Scholar]
- Gerrity R. G., Cliff W. J. The aortic tunica intima in young and aging rats. Exp Mol Pathol. 1972 Jun;16(3):382–402. doi: 10.1016/0014-4800(72)90012-3. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Brown K. D., Birdwell C. R., Zetter B. R. Control of proliferation of human vascular endothelial cells. Characterization of the response of human umbilical vein endothelial cells to fibroblast growth factor, epidermal growth factor, and thrombin. J Cell Biol. 1978 Jun;77(3):774–788. doi: 10.1083/jcb.77.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris I., Majno G. Cellular breakdown within the arterial wall. An ultrastructural study of the coronary artery in young and aging rats. Virchows Arch A Pathol Anat Histol. 1974;364(1):111–127. doi: 10.1007/BF01230861. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Laver-Rudich Z., Skutelsky E., Danon D. Age-related changes in aortic intima of rats. J Gerontol. 1978 May;33(3):337–346. doi: 10.1093/geronj/33.3.337. [DOI] [PubMed] [Google Scholar]
- MARTIN G. M. USE OF TRIS(HYDROXYMETHYL)AMINOMETHANE BUFFERS IN CULTURES OF DIPLOID HUMAN FIBROBLASTS. Proc Soc Exp Biol Med. 1964 May;116:167–171. doi: 10.3181/00379727-116-29191. [DOI] [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Epstein C. J. Replicative life-span of cultivated human cells. Effects of donor's age, tissue, and genotype. Lab Invest. 1970 Jul;23(1):86–92. [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A. Symposium on in vitro studies related to atherogenesis. Life histories of hyperplastoid cell lines from aorta and skin. Exp Mol Pathol. 1973 Apr;18(2):125–141. doi: 10.1016/0014-4800(73)90012-9. [DOI] [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Mayne P. M., Miller E. J. Changes in type of collagen synthesized as clones of chick chondrocytes grow and eventually lose division capacity. Proc Natl Acad Sci U S A. 1976 May;73(5):1674–1678. doi: 10.1073/pnas.73.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. N., Rosen E. M., Levine E. M. Cellular senescence in a cloned strain of bovine fetal aortic endothelial cells. Science. 1980 Feb 22;207(4433):889–891. doi: 10.1126/science.7355268. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhme D. Evidence for a relationship between longevity of mammalian species and life spans of normal fibroblasts in vitro and erythrocytes in vivo. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5009–5013. doi: 10.1073/pnas.78.8.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWIM H. E., PARKER R. F. Culture characteristics of human fibroblasts propagated serially. Am J Hyg. 1957 Sep;66(2):235–243. doi: 10.1093/oxfordjournals.aje.a119897. [DOI] [PubMed] [Google Scholar]
- Sacher G. A., Hart R. W. Longevity, aging and comparative cellular and molecular biology of the house mouse, Mus musculus, and the white-footed mouse, Peromyscus leucopus. Birth Defects Orig Artic Ser. 1978;14(1):71–96. [PubMed] [Google Scholar]
- Schneider E. L., Mitsui Y. The relationship between in vitro cellular aging and in vivo human age. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3584–3588. doi: 10.1073/pnas.73.10.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]






