Abstract
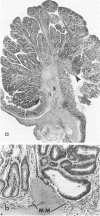
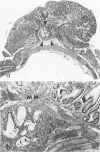
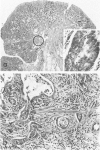
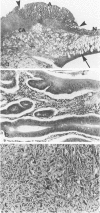
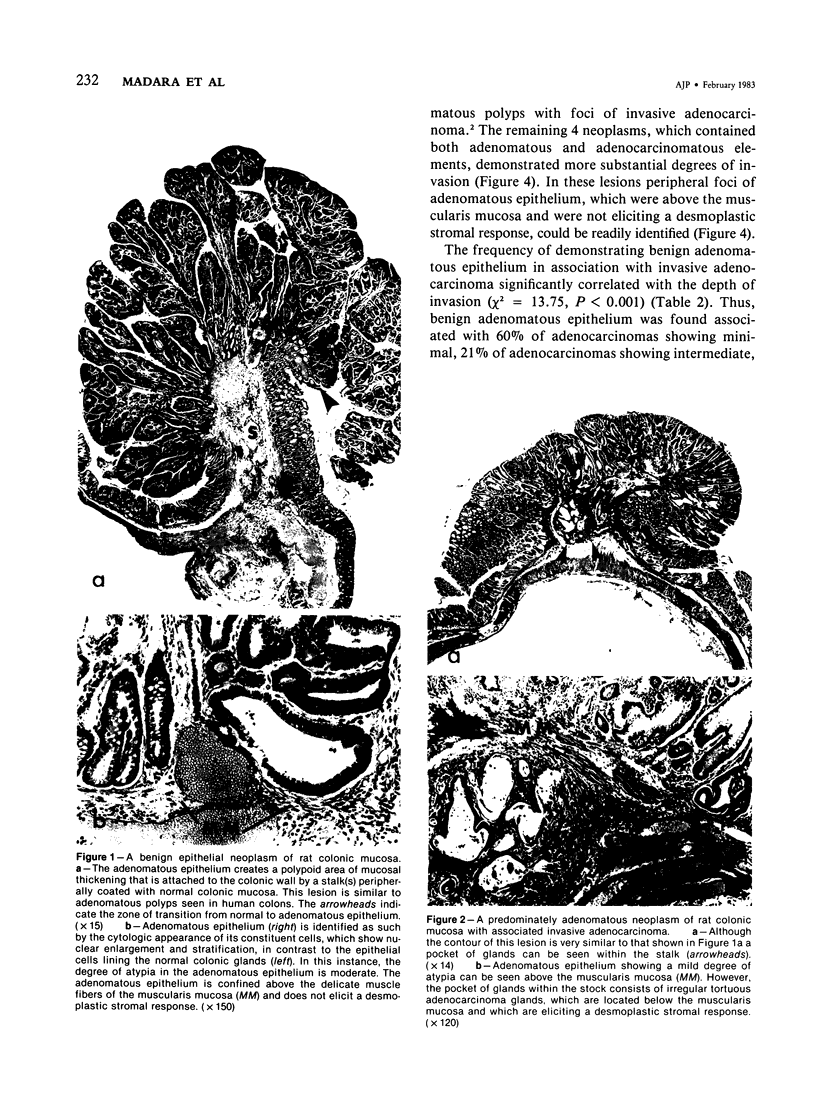
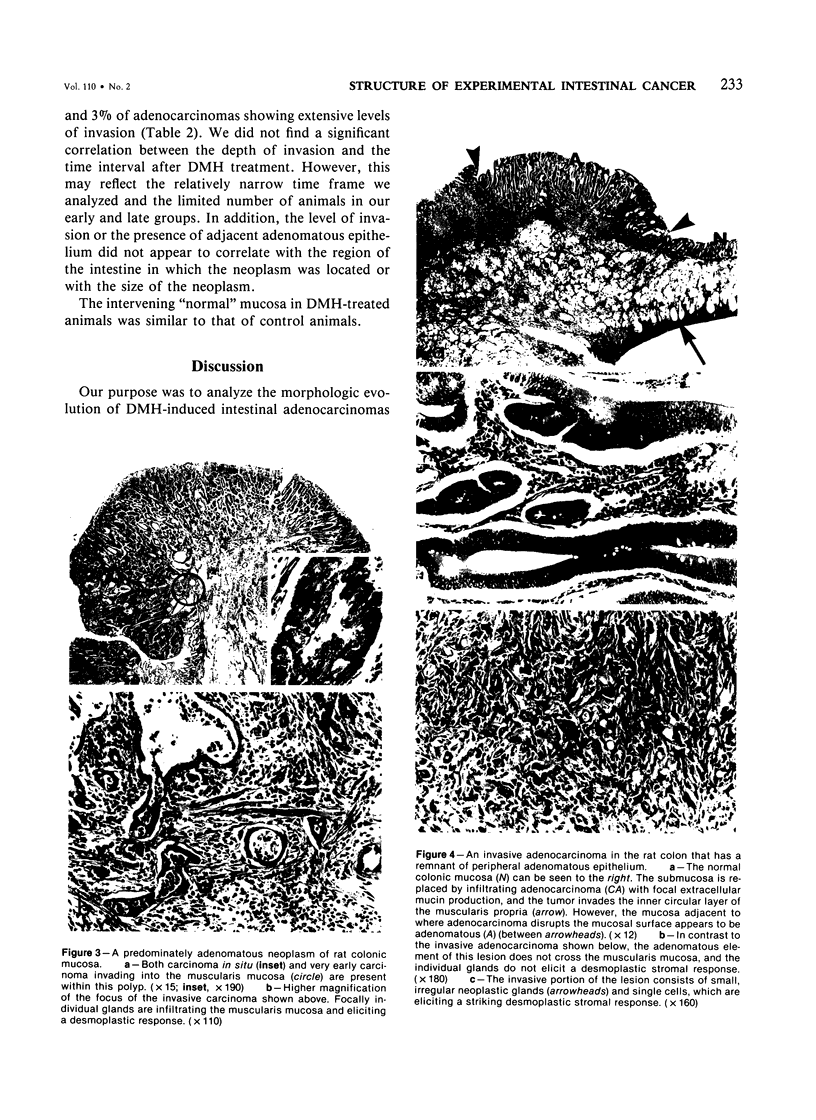
Carcinogen-induced primary intestinal adenocarcinomas serve as a useful animal model for human colonic adenocarcinomas. Although striking similarities between this model and the human disease state exist, there are also troublesome discrepancies-a major one being the reported lack of an adenoma-carcinoma sequence in the experimental model. However, the original morphologic descriptions of these experimental neoplasms predated the development of presently accepted morphologic criteria that have been used to describe the adenoma-carcinoma sequence in humans. Therefore, the authors reevaluated the structural evolution of dimethylhydrazine-induced rat intestinal neoplasms, using the same criteria that were recently applied to evaluate human colonic adenocarcinomas. Such an approach shows that many dimethylhydrazine-induced intestinal adenocarcinomas have peripheral foci of adenomatous epithelium associated with them. In addition, the frequency of this association correlates inversely (P less than .001) with the depth of invasion. These findings are comparable to those which, in humans, have been used as evidence supporting the adenoma-carcinoma sequence. Thus, when assessed with equivalent criteria, dimethylhydrazine-induced intestinal adenocarcinomas appear to be similar, not dissimilar, to human colonic adenocarcinomas in their structural evolution. These data suggest that, at least in part, dimethylhydrazine-induced intestinal adenocarcinomas arise in foci of preexisting adenomatous epithelium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Pollard M., Madsen D. C. Effects of cholestyramine on 1,2-dimethylhydrazine-induced enteric carcinoma in germfree rats. Proc Soc Exp Biol Med. 1975 Dec;150(3):780–785. doi: 10.3181/00379727-150-39124. [DOI] [PubMed] [Google Scholar]
- Asman H. B., Pierce E. R. Familial multiple polyposis. A statistical study of a large Kentucky kindred. Cancer. 1970 Apr;25(4):972–981. doi: 10.1002/1097-0142(197004)25:4<972::aid-cncr2820250435>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Fenoglio C. M., Lane N. The anatomical precursor of colorectal carcinoma. Cancer. 1974 Sep;34(3):suppl–suppl:823. doi: 10.1002/1097-0142(197409)34:3+<819::aid-cncr2820340706>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Gilbertsen V. A. Proctosigmoidoscopy and polypectomy in reducing the incidence of rectal cancer. Cancer. 1974 Sep;34(3):suppl–suppl:939. doi: 10.1002/1097-0142(197409)34:3+<936::aid-cncr2820340722>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- LaMont J. T., O'Gorman T. A. Experimental colon cancer. Gastroenterology. 1978 Dec;75(6):1157–1169. [PubMed] [Google Scholar]
- Lennard-Jones J. E., Morson B. C., Ritchie J. K., Shove D. C., Williams C. B. Cancer in colitis: assessment of the individual risk by clinical and histological criteria. Gastroenterology. 1977 Dec;73(6):1280–1289. [PubMed] [Google Scholar]
- Maskens A. P. Histogenesis and growth pattern of 1,2-dimethylhydrazine-induced rat colon adenocarcinoma. Cancer Res. 1976 May;36(5):1585–1592. [PubMed] [Google Scholar]
- Morson B. C., Pang L. S. Rectal biopsy as an aid to cancer control in ulcerative colitis. Gut. 1967 Oct;8(5):423–434. doi: 10.1136/gut.8.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto T., Bussey H. J., Morson B. C. The evolution of cancer of the colon and rectum. Cancer. 1975 Dec;36(6):2251–2270. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- Nugent F. W., Haggit R. C., Colcher H., Kutteruf G. C. Malignant potential of chronic ulcerative colitis. Preliminary report. Gastroenterology. 1979 Jan;76(1):1–5. [PubMed] [Google Scholar]
- Pozharisski K. M. Morphology and morphogenesis of experimental epithelial tumors of the intestine. J Natl Cancer Inst. 1975 May;54(5):1115–1135. doi: 10.1093/jnci/54.5.1115. [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Narisawa T., Vukusich D., Weisburger J. H., Wynder E. L. Effect of quality and quantity of dietary fat and dimethylhydrazine in colon carcinogenesis in rats. Proc Soc Exp Biol Med. 1976 Feb;151(2):237–239. doi: 10.3181/00379727-151-39181. [DOI] [PubMed] [Google Scholar]
- Shinya H., Wolff W. I. Morphology, anatomic distribution and cancer potential of colonic polyps. Ann Surg. 1979 Dec;190(6):679–683. doi: 10.1097/00000658-197912000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele G., Jr, Harte P. J., Rayner A. A., Corson J. M., Madara J., Munroe A. E., King V. P., Wilson R. E. The effect of adjuvant immunotherapy on tumor recurrence after segmental resection of carcinogen-induced Wistar/Furth primary bowel adenocarcinomas. J Immunol. 1982 Jan;128(1):7–10. [PubMed] [Google Scholar]
- Steele G., Jr, Sjogren H. O. Cell surface antigens in a rat colon cancer model: correlation with inhibition of tumor growth. Surgery. 1977 Jul;82(1):164–169. [PubMed] [Google Scholar]
- Ward J. M. Morphogenesis of chemically induced neoplasms of the colon and small intestine in rats. Lab Invest. 1974 Apr;30(4):505–513. [PubMed] [Google Scholar]
- Yardley J. H., Keren D. F. "Precancer" lesions in ulcerative colits. A retrospective study of rectal biopsy and colectomy specimens. Cancer. 1974 Sep;34(3):suppl–suppl:844. doi: 10.1002/1097-0142(197409)34:3+<835::aid-cncr2820340709>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]