Abstract
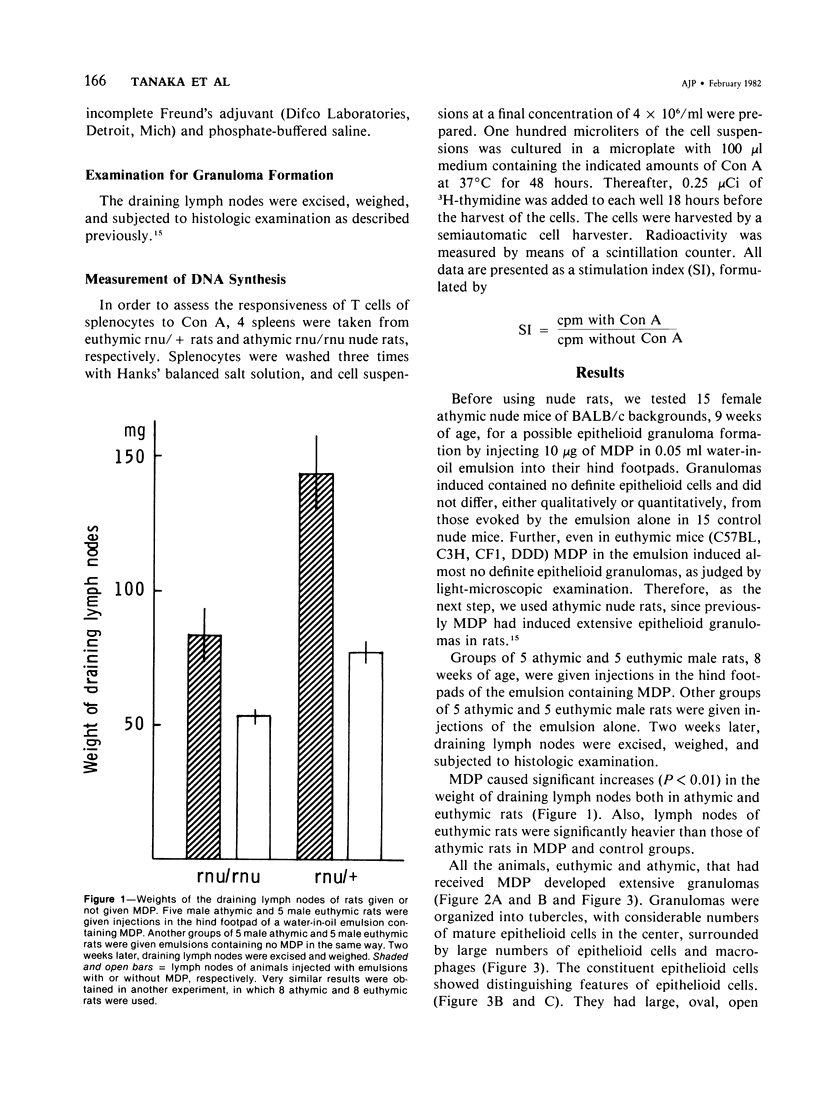
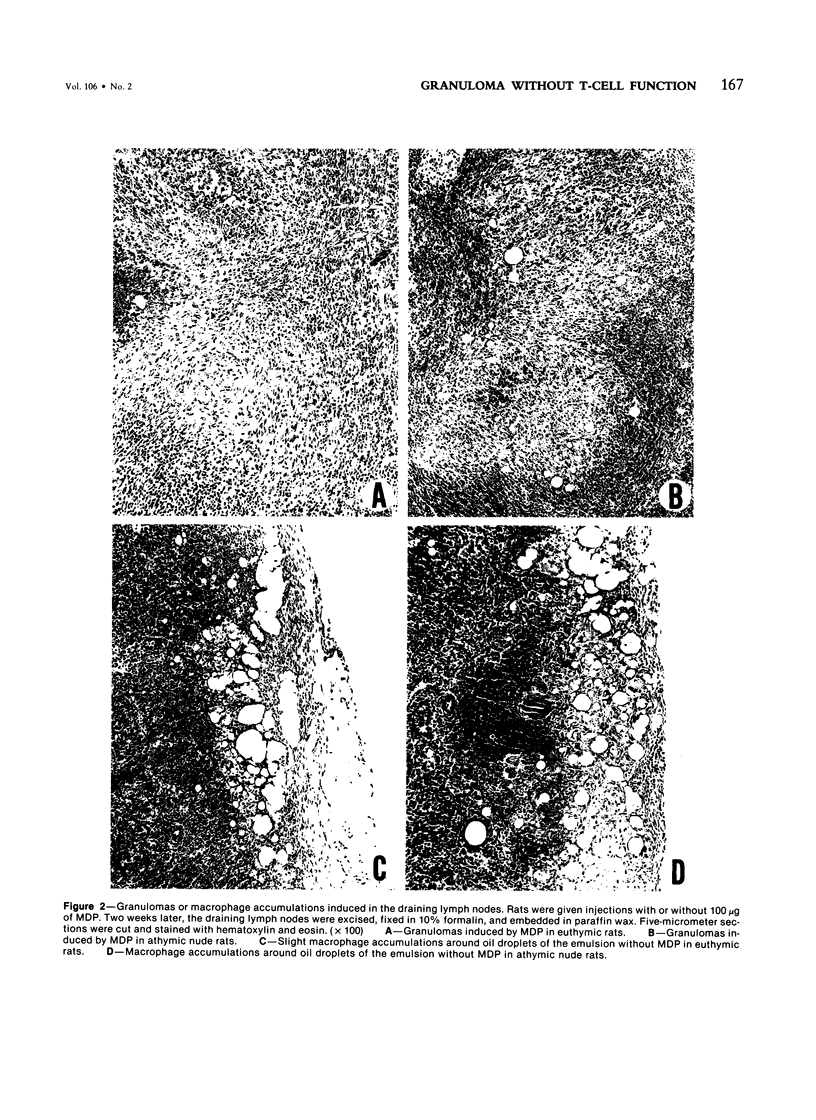
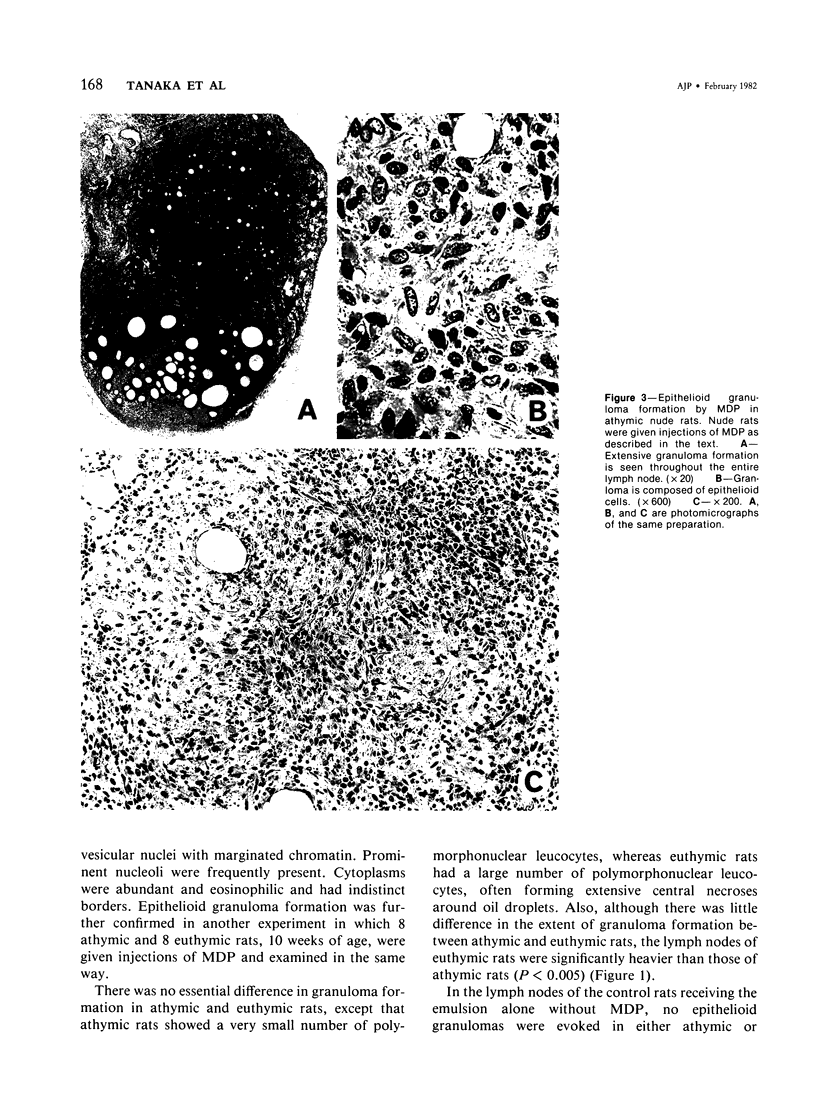
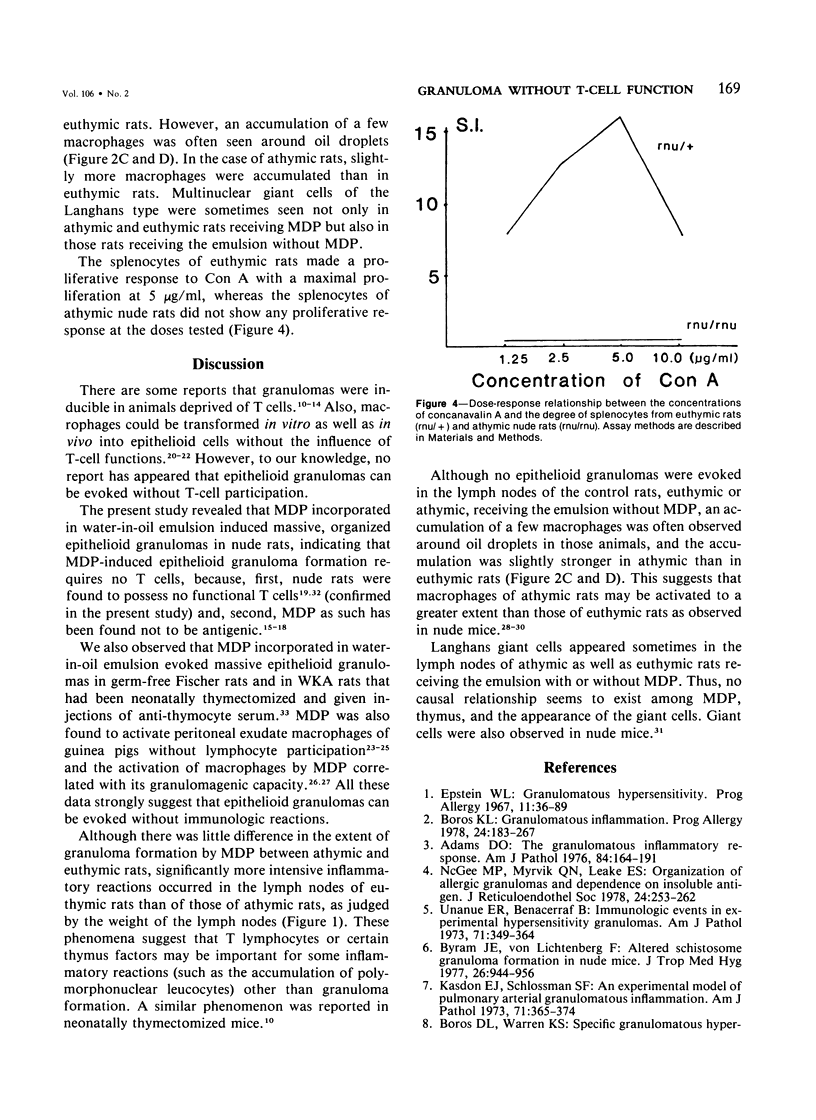
Muramyl dipeptide (MDP), a minimal structure in bacterial cell walls essential for their adjuvant activity, was incorporated in a water-in-oil emulsion and injected into the footpads of nude rats devoid of functional T cells. MDP thus injected evoked massive epithelioid granulomas in the draining lymph nodes, indicating that MDP induced epithelioid granuloma formation requires no T cells. This finding with other data available strongly suggest that epithelioid granulomas can be induced without immunologic reactions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Amsden A. F., Boros D. L., Hood A. T. Etiology of the liver granulomatous response in Schistosoma mansoni-infected athymic nude mice. Infect Immun. 1980 Jan;27(1):75–80. doi: 10.1128/iai.27.1.75-80.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros D. L. Granulomatous inflammations. Prog Allergy. 1978;24:183–267. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Specific granulomatous hypersensitivity elicited by bentonite particles coated with soluble antigens from schistosome eggs and turcle bacilli. Nature. 1971 Jan 15;229(5281):200–201. doi: 10.1038/229200a0. [DOI] [PubMed] [Google Scholar]
- Byram J. E., von Lichtenberg F. Altered schistosome granuloma formation in nude mice. Am J Trop Med Hyg. 1977 Sep;26(5 Pt 1):944–956. doi: 10.4269/ajtmh.1977.26.944. [DOI] [PubMed] [Google Scholar]
- Chedid L., Audibert F., Johnson A. G. Biological activities of muramyl dipeptide, a synthetic glycopeptide analogous to bacterial immunoregulating agents. Prog Allergy. 1978;25:63–105. [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Crawford C. L., Hardwicke P. M., Evans D. H., Evans E. M. Granulomatous hypersensitivity induced by sensory peripheral nerve. Nature. 1977 Feb 3;265(5593):457–459. doi: 10.1038/265457a0. [DOI] [PubMed] [Google Scholar]
- Emori K., Tanaka A. Granuloma formation by synthetic bacterial cell wall fragment: muramyl dipeptide. Infect Immun. 1978 Feb;19(2):613–620. doi: 10.1128/iai.19.2.613-620.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W. L., Fukuyama K., Danno K., Kwan-Wong E. Granulomatous inflammation in normal and athymic mice infected with schistosoma mansoni: an ultrastructural study. J Pathol. 1979 Apr;127(4):207–215. doi: 10.1002/path.1711270408. [DOI] [PubMed] [Google Scholar]
- Epstein W. L. Granulomatous hypersensitivity. Prog Allergy. 1967;11:36–88. [PubMed] [Google Scholar]
- Heymer B., Hobik H. P., Schäfer H., Bültmann B., Spanel R., Haferkamp O. Animal experimental studies on chronic granulomatous inflammation and T-lymphocyte-system. Beitr Pathol. 1975 Nov;156(2):128–144. doi: 10.1016/s0005-8165(75)80146-6. [DOI] [PubMed] [Google Scholar]
- Kasdon E. J., Schlossman S. F. An experimental model of pulmonary arterial granulomatous inflammation. Am J Pathol. 1973 Jun;71(3):365–372. [PMC free article] [PubMed] [Google Scholar]
- Machado E. A., Lair S. V. Giant multinucleate macrophages in methyl cellulose-stimulated athymic nude mice. J Reticuloendothel Soc. 1978 May;23(5):383–387. [PubMed] [Google Scholar]
- McGee M. P., Myrvik Q. N., Leake E. S. Organization of allergic granulomas and dependence on insoluble antigen. J Reticuloendothel Soc. 1978 Sep;24(3):253–262. [PubMed] [Google Scholar]
- Mokhtar N. M., Spector W. G. Facsimile epithelioid cells obtained from stimulated peritoneal macrophages and their secretory activity in vitro. J Pathol. 1979 Jul;128(3):117–126. doi: 10.1002/path.1711280302. [DOI] [PubMed] [Google Scholar]
- Nagao S., Miki T., Tanaka A. Macrophage activation by muramyl dipeptide (MDP) without lymphocyte participation. Microbiol Immunol. 1981;25(1):41–50. doi: 10.1111/j.1348-0421.1981.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Nagao S., Tanaka A., Yamamoto Y., Koga T., Onoue K., Shiba T., Kusumoto K., Kotani S. Inhibition of macrophage migration by muramyl peptides. Infect Immun. 1979 May;24(2):308–312. doi: 10.1128/iai.24.2.308-312.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou J. M., Spector W. G. The origin, properties and fate of epithelioid cells. J Pathol. 1971 Nov;105(3):187–203. doi: 10.1002/path.1711050305. [DOI] [PubMed] [Google Scholar]
- Rao G. R., Rawls W. E., Perey D. Y., Tompkins W. A. Macrophage activation in congenitally athymic mice raised under conventional or germ-free conditions. J Reticuloendothel Soc. 1977 Jan;21(1):13–20. [PubMed] [Google Scholar]
- Rothwell T. L., Spector W. G. The effect of neonatal and adult thymectomy on the inflammatory response. J Pathol. 1972 Sep;108(1):15–21. doi: 10.1002/path.1711080103. [DOI] [PubMed] [Google Scholar]
- Sutton J. S., Weiss L. Transformation of monocytes in tissue culture into macrophages, epithelioid cells, and multinucleated giant cells. An electron microscope study. J Cell Biol. 1966 Feb;28(2):303–332. doi: 10.1083/jcb.28.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya K., Shimotori S., Taniguchi T., Nomoto K. Cellular mechanisms in the protection against infection by Listeria monocytogenes in mice. J Gen Microbiol. 1977 Jun;100(2):373–379. doi: 10.1099/00221287-100-2-373. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Emori K. Epithelioid granuloma formation by a synthetic bacterial cell wall component, muramyl dipeptide (MDP). Am J Pathol. 1980 Mar;98(3):733–748. [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Nagao S., Imai K., Mori R. Macrophage activation by muramyl dipeptide as measured by macrophage spreading and attachment. Microbiol Immunol. 1980;24(6):547–557. doi: 10.1111/j.1348-0421.1980.tb02858.x. [DOI] [PubMed] [Google Scholar]
- Terada E., Nakayama T., Hioki K., Saito M., Okudaira H. [Absence of T lymphocyte functions in athymic nude rats (author's transl)]. Jikken Dobutsu. 1980 Jul;29(3):365–367. doi: 10.1538/expanim1978.29.3_365. [DOI] [PubMed] [Google Scholar]
- Ueda K., Yamazaki S., Someya S. Experimental mycobacterial infection in congenitally athymic "nude" mice. J Reticuloendothel Soc. 1976 Feb;19(2):77–90. [PubMed] [Google Scholar]
- Unanue E. R., Benacerraf B. Immunologic events in experimental hypersensitivity granulomas. Am J Pathol. 1973 Jun;71(3):349–364. [PMC free article] [PubMed] [Google Scholar]
- Vos J. G., Kreeftenberg J. G., Kruijt B. C., Kruizinga W., Steerenberg P. The athymic nude rat. II. Immunological characteristics. Clin Immunol Immunopathol. 1980 Feb;15(2):229–237. doi: 10.1016/0090-1229(80)90033-1. [DOI] [PubMed] [Google Scholar]