Abstract
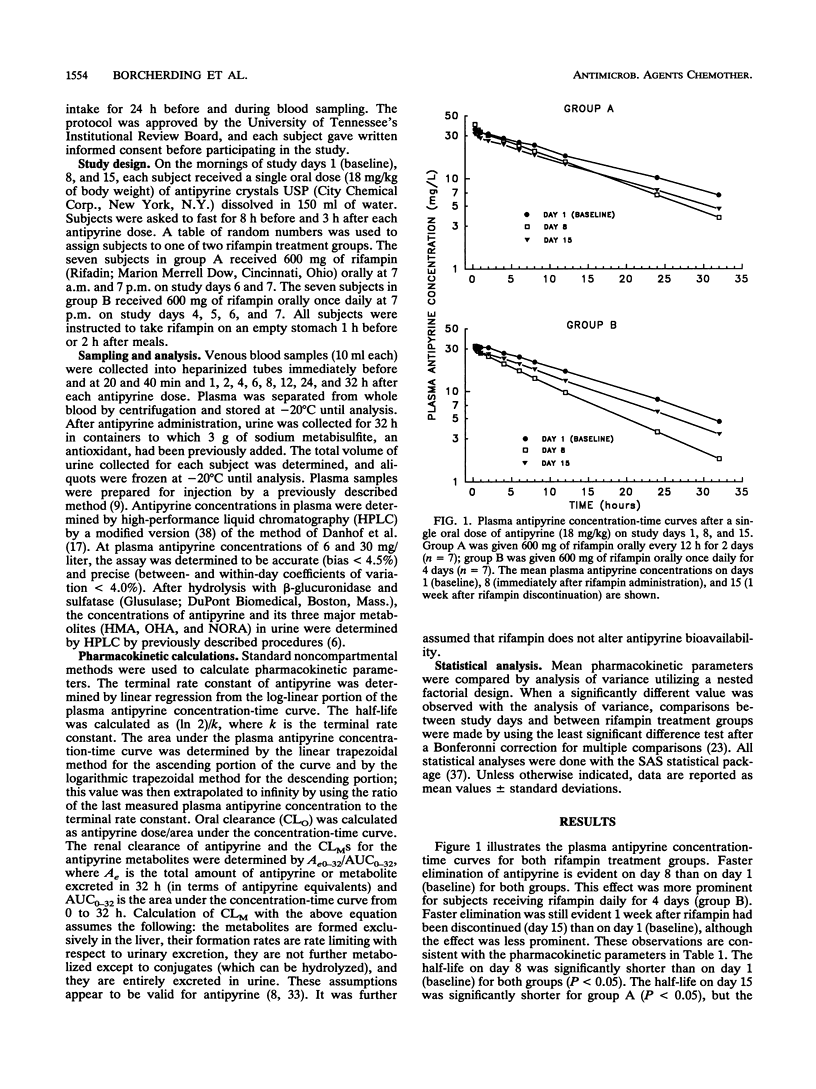
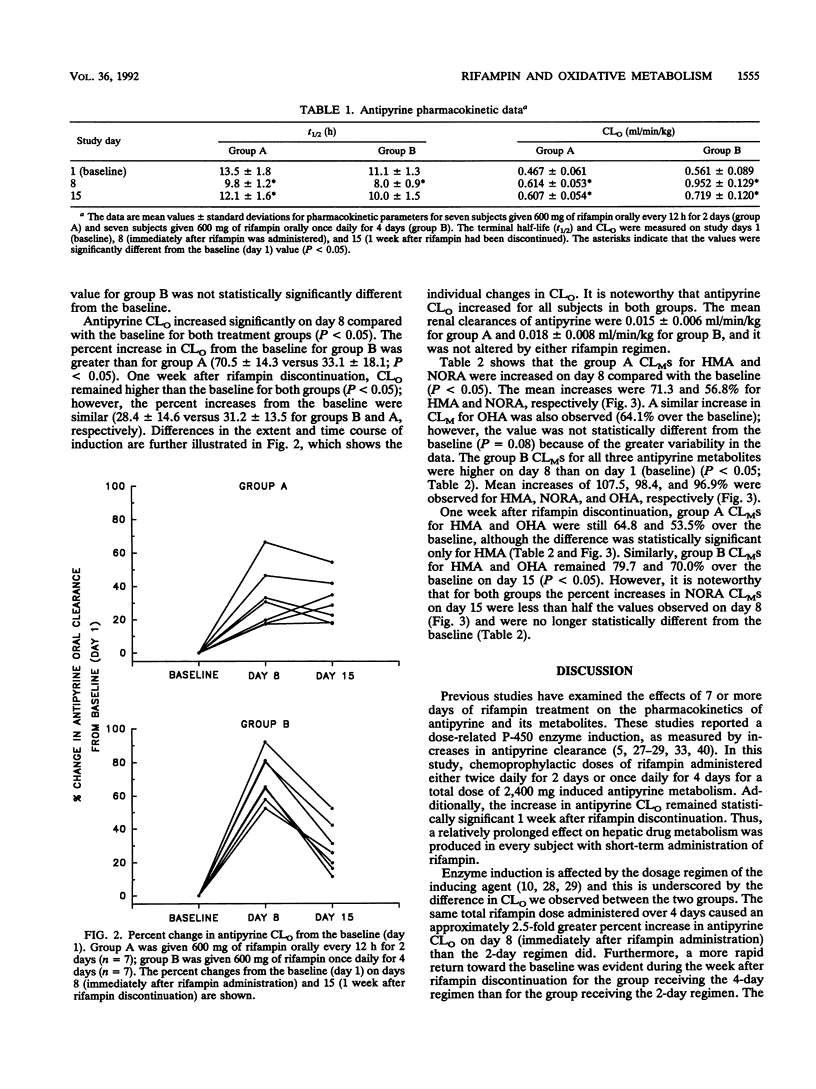
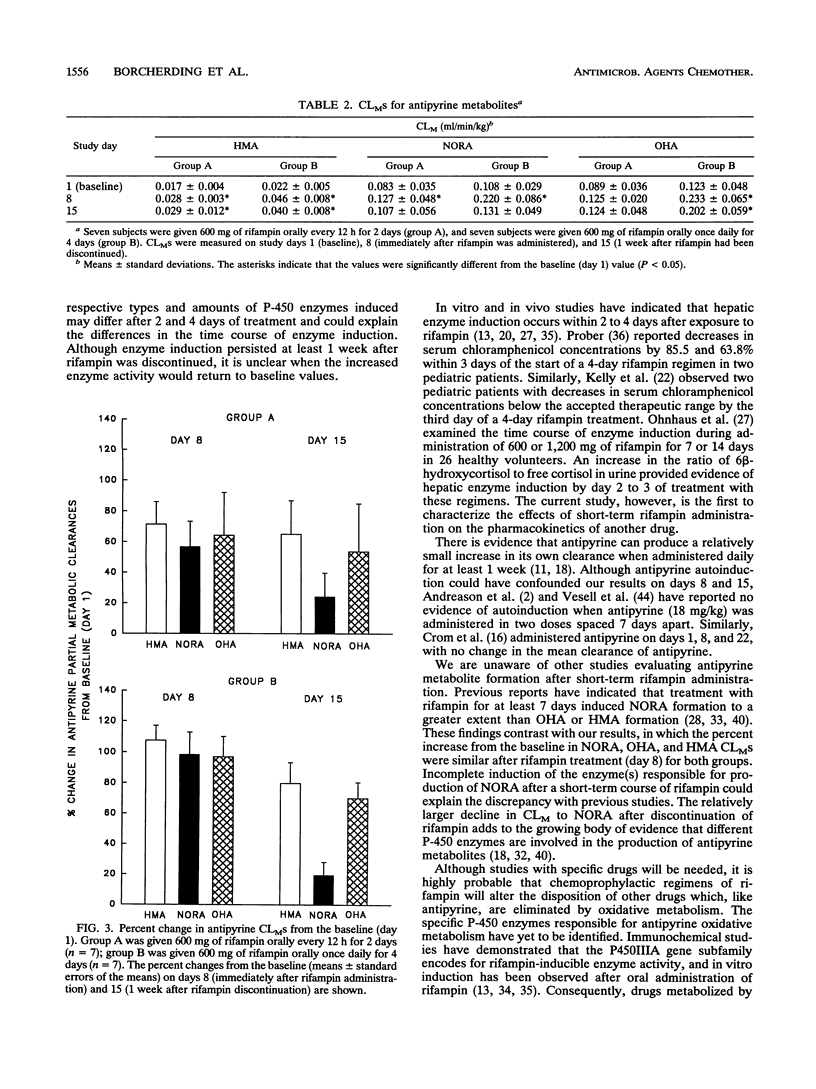
The effects of two short-term chemoprophylaxis regimens of rifampin (2 or 4 days) on oxidative metabolism were investigated in 14 healthy subjects. Seven subjects received 600 mg of rifampin twice daily on study days 6 and 7 (group A), and seven subjects received 600 mg of rifampin once daily on days 4, 5, 6, and 7 (group B). Antipyrine (18 mg/kg of body weight) was administered orally on days 1, 8, and 15. Short-term rifampin regimens increased oral clearance of antipyrine in both groups compared with the baseline value (P less than 0.05), and group B displayed a larger percent increase over the baseline value than group A did (70.5 +/- 14.3 versus 33.1 +/- 18.1; P less than 0.05). The partial metabolic clearance (CLM) of antipyrine to 3-hydroxymethylantipyrine (HMA) on day 8 increased 71 and 108% for regimens A and B, respectively (P less than 0.05 for both). The corresponding increases in CLM to norantipyrine (NORA) were 57 and 98% (P less than 0.05 for both). CLM to 4-hydroxyantipyrine (OHA) on day 8 increased 64% for regimen A (P = 0.08) and 97% for regimen B (P less than 0.05) compared with the baseline. Although CLM to HMA and OHA on day 15 remained greater than 50% over the baseline with both regimens, CLM to NORA on day 15 was less than 25% over the baseline with both regimens. Thus, both short-term rifampin chemoprophylaxis regimens increased antipyrine clearance for at least 1 week. The increase tended to be higher with the 4-day regimen. The pattern observed for the CLMS suggests that more than one P-450 enzyme is affected.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Hunnisett A. G., Morris P. J. Cyclosporin and rifampicin in renal transplantation. Lancet. 1985 Apr 27;1(8435):980–980. doi: 10.1016/s0140-6736(85)91746-5. [DOI] [PubMed] [Google Scholar]
- Andreasen P. B., Vesell E. S. Comparison of plasma levels of antipyrine, tolbutamide, and warfarin after oral and intravenous administration. Clin Pharmacol Ther. 1974 Dec;16(6):1059–1065. doi: 10.1002/cpt19741661059. [DOI] [PubMed] [Google Scholar]
- Baciewicz A. M., Self T. H., Bekemeyer W. B. Update on rifampin drug interactions. Arch Intern Med. 1987 Mar;147(3):565–568. [PubMed] [Google Scholar]
- Baciewicz A. M., Self T. H. Rifampin drug interactions. Arch Intern Med. 1984 Aug;144(8):1667–1671. doi: 10.1001/archinte.144.8.1667. [DOI] [PubMed] [Google Scholar]
- Bennett P. N., John V. A., Whitmarsh V. B. Effect of rifampicin on metoprolol and antipyrine kinetics. Br J Clin Pharmacol. 1982 Mar;13(3):387–391. doi: 10.1111/j.1365-2125.1982.tb01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyden G. T., LeDuc B. W., Greenblatt D. J. Large theophylline requirements due to high theophylline clearance: verification by the antipyrine test. Pharmacology. 1986;32(4):226–231. doi: 10.1159/000138173. [DOI] [PubMed] [Google Scholar]
- Bolt H. M., Kappus H., Bolt M. Effect of rifampicin treatment on the metabolism of oestradiol and 17alpha-ethinyloestradiol by human liver microsomes. Eur J Clin Pharmacol. 1975 Jun 13;8(5):301–307. doi: 10.1007/BF00562654. [DOI] [PubMed] [Google Scholar]
- Boobis A. R., Brodie M. J., Kahn G. C., Toverud E. L., Blair I. A., Murray S., Davies D. S. Comparison of the in vivo and in vitro rates of formation of the three main oxidative metabolites of antipyrine in man. Br J Clin Pharmacol. 1981 Dec;12(6):771–777. doi: 10.1111/j.1365-2125.1981.tb01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottorff M. B., Lalonde R. L., Kazierad D. J., Hoon T. J., Tsiu S. J., Mirvis D. M. The effects of encainide versus diltiazem on the oxidative metabolic pathways of antipyrine. Pharmacotherapy. 1989;9(5):315–321. doi: 10.1002/j.1875-9114.1989.tb04143.x. [DOI] [PubMed] [Google Scholar]
- Branch R. A., Herman R. J. Enzyme induction and beta-adrenergic receptor blocking drugs. Br J Clin Pharmacol. 1984;17 (Suppl 1):77S–84S. doi: 10.1111/j.1365-2125.1984.tb02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge A., Orme M. L., Thorgeirsson S., Davies D. S., Brooks R. V. Drug interactions with warfarin: studies with dichloralphenazone, chloral hydrate and phenazone (antipyrine). Clin Sci. 1971 Apr;40(4):351–364. doi: 10.1042/cs0400351. [DOI] [PubMed] [Google Scholar]
- Combalbert J., Fabre I., Fabre G., Dalet I., Derancourt J., Cano J. P., Maurel P. Metabolism of cyclosporin A. IV. Purification and identification of the rifampicin-inducible human liver cytochrome P-450 (cyclosporin A oxidase) as a product of P450IIIA gene subfamily. Drug Metab Dispos. 1989 Mar-Apr;17(2):197–207. [PubMed] [Google Scholar]
- Crom W. R., Webster S. L., Bobo L., Teresi M. E., Relling M. V., Evans W. E. Simultaneous administration of multiple model substrates to assess hepatic drug clearance. Clin Pharmacol Ther. 1987 Jun;41(6):645–650. doi: 10.1038/clpt.1987.90. [DOI] [PubMed] [Google Scholar]
- Danhof M., Groot-van der Vis E., Breiner D. D. Assay of antipyrine and its primary metabolites in plasma, saliva and urine by high-performance liquid chromatography and some preliminary results in man. Pharmacology. 1979;18(4):210–223. doi: 10.1159/000137254. [DOI] [PubMed] [Google Scholar]
- Danhof M., Verbeek R. M., van Boxtel C. J., Boeijinga J. K., Breimer D. D. Differential effects of enzyme induction on antipyrine metabolite formation. Br J Clin Pharmacol. 1982 Mar;13(3):379–386. doi: 10.1111/j.1365-2125.1982.tb01389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelbaum M., Sonntag B., Dengler H. J. HPLC determination of antipyrine metabolites. Pharmacology. 1981;23(4):192–202. doi: 10.1159/000137550. [DOI] [PubMed] [Google Scholar]
- Ged C., Rouillon J. M., Pichard L., Combalbert J., Bressot N., Bories P., Michel H., Beaune P., Maurel P. The increase in urinary excretion of 6 beta-hydroxycortisol as a marker of human hepatic cytochrome P450IIIA induction. Br J Clin Pharmacol. 1989 Oct;28(4):373–387. doi: 10.1111/j.1365-2125.1989.tb03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezequel A. M., Orlandi F., Tenconi L. T. Changes of the smooth endoplasmic reticulum induced by rifampicin in human and guinea-pig hepatocytes. Gut. 1971 Dec;12(12):984–987. doi: 10.1136/gut.12.12.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly H. W., Couch R. C., Davis R. L., Cushing A. H., Knott R. Interaction of chloramphenicol and rifampin. J Pediatr. 1988 May;112(5):817–820. doi: 10.1016/s0022-3476(88)83216-5. [DOI] [PubMed] [Google Scholar]
- LaForce F. M. Immunizations, immunoprophylaxis, and chemoprophylaxis to prevent selected infections. US Preventive Services Task Force. JAMA. 1987 May 8;257(18):2464–2470. [PubMed] [Google Scholar]
- Modry D. L., Stinson E. B., Oyer P. E., Jamieson S. W., Baldwin J. C., Shumway N. E. Acute rejection and massive cyclosporine requirements in heart transplant recipients treated with rifampin. Transplantation. 1985 Mar;39(3):313–314. doi: 10.1097/00007890-198503000-00022. [DOI] [PubMed] [Google Scholar]
- Ohnhaus E. E., Breckenridge A. M., Park B. K. Urinary excretion of 6 beta-hydroxycortisol and the time course measurement of enzyme induction in man. Eur J Clin Pharmacol. 1989;36(1):39–46. doi: 10.1007/BF00561021. [DOI] [PubMed] [Google Scholar]
- Ohnhaus E. E., Brockmeyer N., Dylewicz P., Habicht H. The effect of antipyrine and rifampin on the metabolism of diazepam. Clin Pharmacol Ther. 1987 Aug;42(2):148–156. doi: 10.1038/clpt.1987.125. [DOI] [PubMed] [Google Scholar]
- Ohnhaus E. E., Park B. K. Measurement of urinary 6-beta-hydroxycortisol excretion as an in vivo parameter in the clinical assessment of the microsomal enzyme-inducing capacity of antipyrine, phenobarbitone and rifampicin. Eur J Clin Pharmacol. 1979 Mar 26;15(2):139–145. doi: 10.1007/BF00609878. [DOI] [PubMed] [Google Scholar]
- Okey A. B. Enzyme induction in the cytochrome P-450 system. Pharmacol Ther. 1990;45(2):241–298. doi: 10.1016/0163-7258(90)90030-6. [DOI] [PubMed] [Google Scholar]
- Park B. K., Breckenridge A. M. Clinical implications of enzyme induction and enzyme inhibition. Clin Pharmacokinet. 1981 Jan-Feb;6(1):1–24. doi: 10.2165/00003088-198106010-00001. [DOI] [PubMed] [Google Scholar]
- Park B. K., Kitteringham N. R. Assessment of enzyme induction and enzyme inhibition in humans: toxicological implications. Xenobiotica. 1990 Nov;20(11):1171–1185. doi: 10.3109/00498259009046837. [DOI] [PubMed] [Google Scholar]
- Perucca E., Grimaldi R., Frigo G. M., Sardi A., Mönig H., Ohnhaus E. E. Comparative effects of rifabutin and rifampicin on hepatic microsomal enzyme activity in normal subjects. Eur J Clin Pharmacol. 1988;34(6):595–599. doi: 10.1007/BF00615223. [DOI] [PubMed] [Google Scholar]
- Pichard L., Fabre I., Fabre G., Domergue J., Saint Aubert B., Mourad G., Maurel P. Cyclosporin A drug interactions. Screening for inducers and inhibitors of cytochrome P-450 (cyclosporin A oxidase) in primary cultures of human hepatocytes and in liver microsomes. Drug Metab Dispos. 1990 Sep-Oct;18(5):595–606. [PubMed] [Google Scholar]
- Pichard L., Gillet G., Fabre I., Dalet-Beluche I., Bonfils C., Thenot J. P., Maurel P. Identification of the rabbit and human cytochromes P-450IIIA as the major enzymes involved in the N-demethylation of diltiazem. Drug Metab Dispos. 1990 Sep-Oct;18(5):711–719. [PubMed] [Google Scholar]
- Prober C. G. Effect of rifampin on chloramphenicol levels. N Engl J Med. 1985 Mar 21;312(12):788–789. doi: 10.1056/NEJM198503213121212. [DOI] [PubMed] [Google Scholar]
- Schellens J. H., van der Wart J. H., Danhof M., van der Velde E. A., Breimer D. D. Relationship between the metabolism of antipyrine, hexobarbitone and theophylline in man as assessed by a 'cocktail' approach. Br J Clin Pharmacol. 1988 Oct;26(4):373–384. doi: 10.1111/j.1365-2125.1988.tb03394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen M. W., De Leede L. G., Boeijinga J. K., Breimer D. D. Correlation between antipyrine metabolite formation and theophylline metabolism in humans after simultaneous single-dose administration and at steady state. J Pharmacol Exp Ther. 1985 Jun;233(3):770–775. [PubMed] [Google Scholar]
- Toverud E. L., Boobis A. R., Brodie M. J., Murray S., Bennett P. N., Whitmarsh V., Davies D. S. Differential induction of antipyrine metabolism by rifampicin. Eur J Clin Pharmacol. 1981;21(2):155–160. doi: 10.1007/BF00637517. [DOI] [PubMed] [Google Scholar]
- Van Buren D., Wideman C. A., Ried M., Gibbons S., Van Buren C. T., Jarowenko M., Flechner S. M., Frazier O. H., Cooley D. A., Kahan B. D. The antagonistic effect of rifampin upon cyclosporine bioavailability. Transplant Proc. 1984 Dec;16(6):1642–1645. [PubMed] [Google Scholar]
- Vesell E. S., Passananti G. T., Glenwright P. A., Dvorchik B. H. Studies on the disposition of antipyrine, aminopyrine, and phenacetin using plasma, saliva, and urine. Clin Pharmacol Ther. 1975 Sep;18(3):259–272. doi: 10.1002/cpt1975183259. [DOI] [PubMed] [Google Scholar]
- Vesell E. S., Shively C. A., Passananti G. T. Failure of cola nut chewing to alter antipyrine disposition in normal male subjects from a small town in South Central Pennsylvania. Clin Pharmacol Ther. 1979 Sep;26(3):287–293. doi: 10.1002/cpt1979263287. [DOI] [PubMed] [Google Scholar]
- Vesell E. S. The antipyrine test in clinical pharmacology: conceptions and misconceptions. Clin Pharmacol Ther. 1979 Sep;26(3):275–286. doi: 10.1002/cpt1979263275. [DOI] [PubMed] [Google Scholar]


