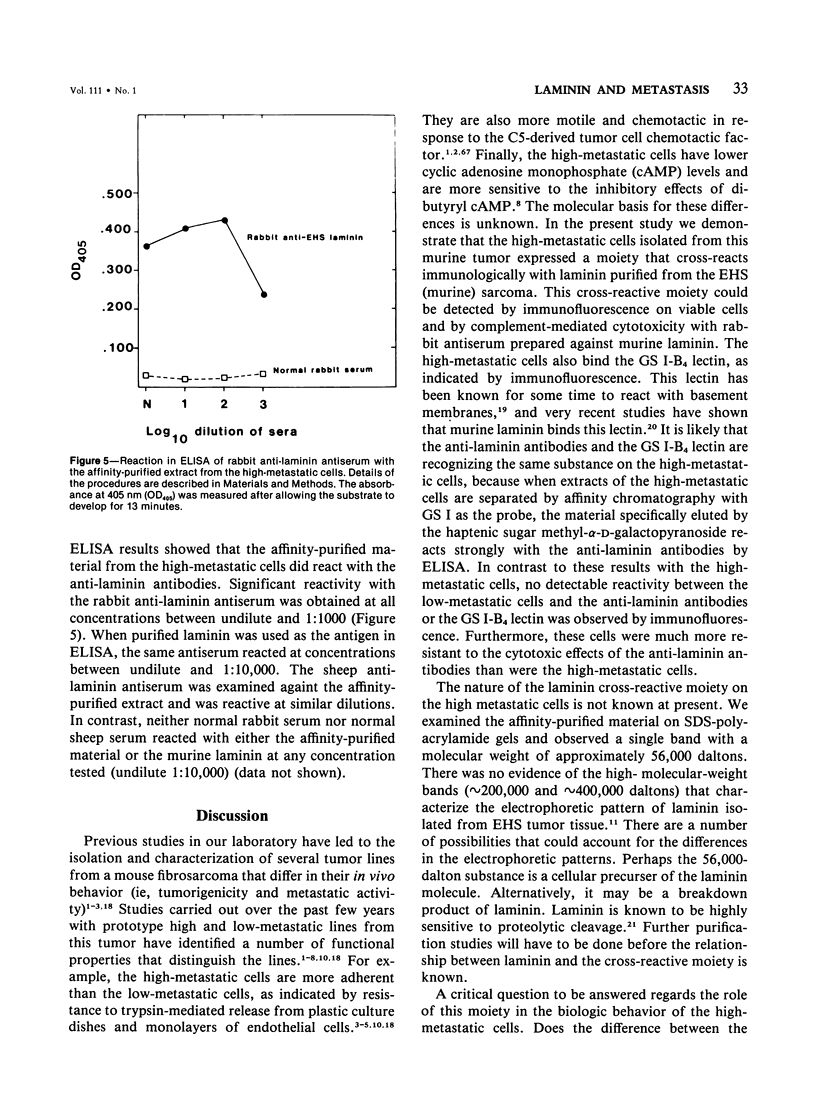
Abstract
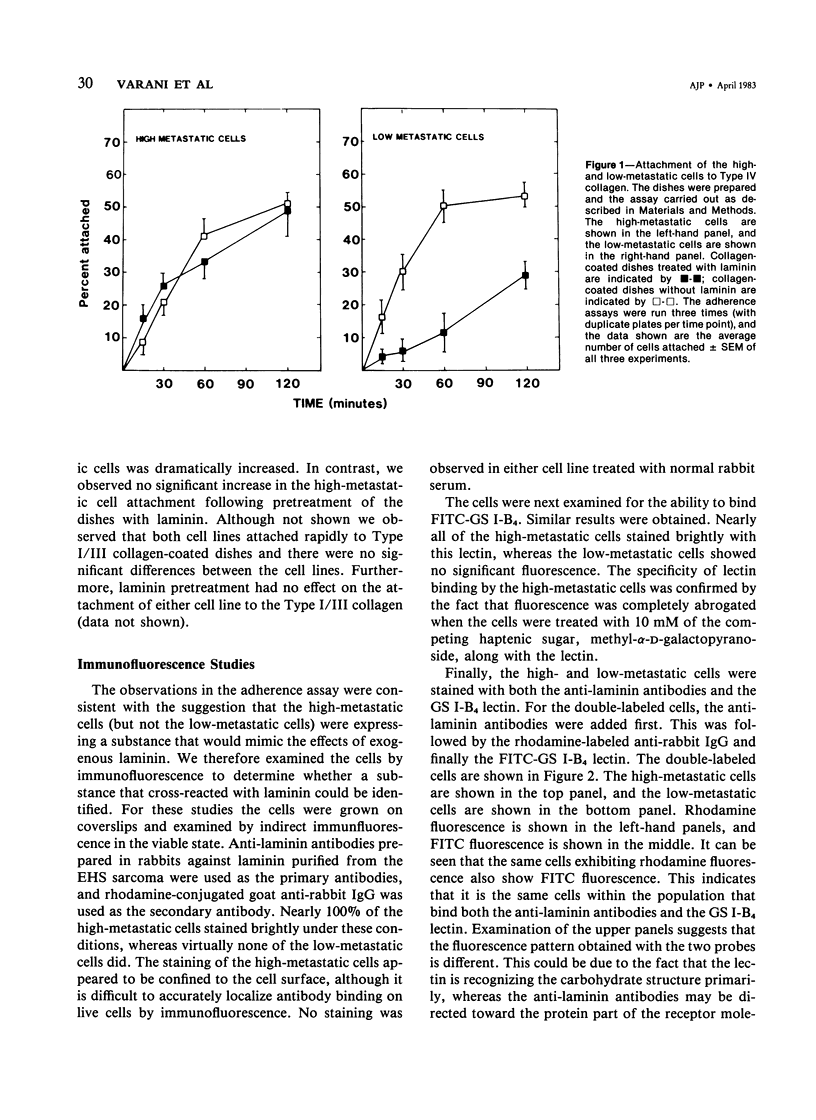
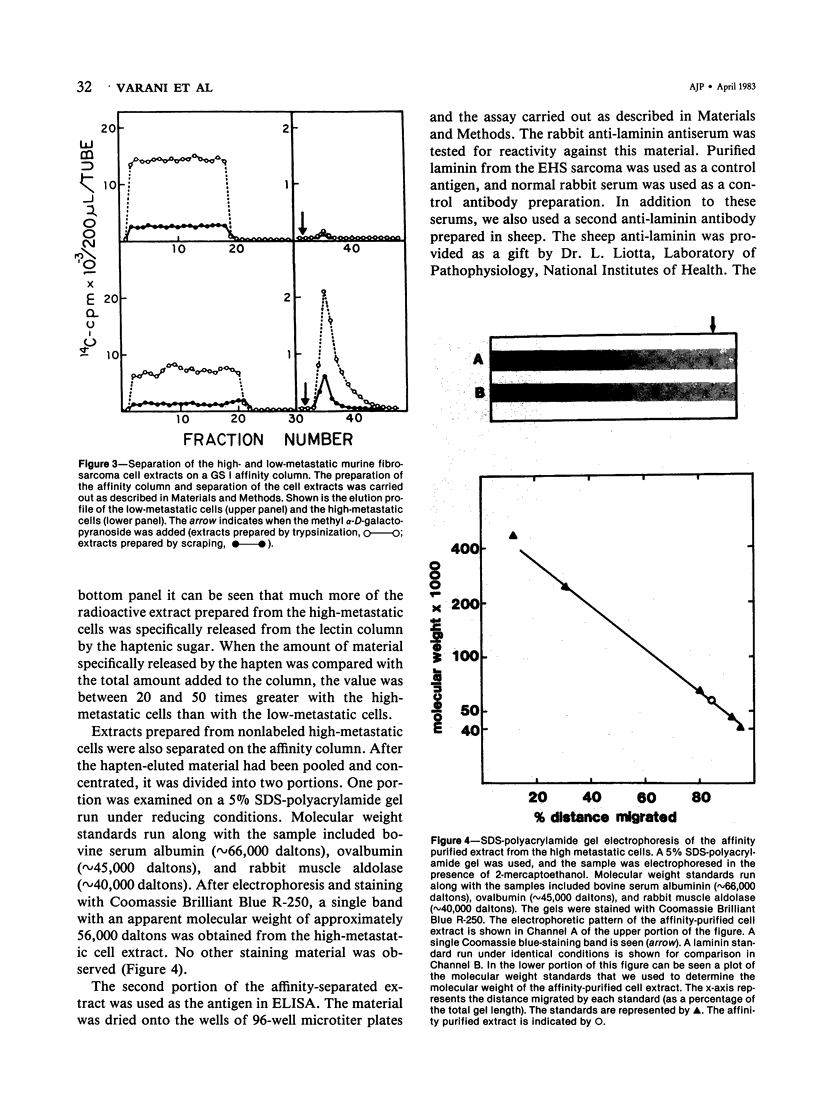
High-metastatic murine fibrosarcoma cells readily attached to Type IV (basement membrane) collagen, whereas low-metastatic cells isolated from the same tumor did not. The addition of laminin--a glycoprotein that facilitates the adherence of epithelial cells to their basement membranes--enhanced the attachment of the low-metastatic cells, but not the high-metastatic cells. Using anti-laminin antibodies and a laminin-binding lectin as probes, the authors were able to identify by immunofluorescence a moiety associated with the high-metastatic cells, but not the low-metastatic cells, which cross-reacted with murine laminin purified from the EHS sarcoma. When extracts from the high-metastatic cells were separated by affinity chromatography, with the laminin-binding lectin as the affinity substrate, a substance was isolated that had an apparent molecular weight of 56,000 daltons. The affinity-purified material reacted strongly with anti-laminin antibodies by enzyme-linked immunosorbent assay.
Full text
PDF




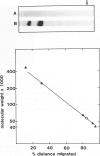
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elgebaly S., Kunkel S., Lovett E. J., Varani J. CAMP differences between clones of high and low malignant fibrosarcoma cells. Oncology. 1982;39(3):163–166. doi: 10.1159/000225629. [DOI] [PubMed] [Google Scholar]
- Hayes C. E., Goldstein I. J. An alpha-D-galactosyl-binding lectin from Bandeiraea simplicifolia seeds. Isolation by affinity chromatography and characterization. J Biol Chem. 1974 Mar 25;249(6):1904–1914. [PubMed] [Google Scholar]
- Klebe R. J. Isolation of a collagen-dependent cell attachment factor. Nature. 1974 Jul 19;250(463):248–251. doi: 10.1038/250248a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Goldfarb R. H., Brundage R., Siegal G. P., Terranova V., Garbisa S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981 Nov;41(11 Pt 1):4629–4636. [PubMed] [Google Scholar]
- Michaelson J. H., McCoy J. P., Jr, Bigazzi P. E. Antibodies to glomerular basement membrane: detection by an enzyme-linked immunosorbent assay in the sera of Brown Norway rats. Kidney Int. 1981 Aug;20(2):285–288. doi: 10.1038/ki.1981.133. [DOI] [PubMed] [Google Scholar]
- Orr F. W., Varani J., Delikatny J., Jain N., Ward P. A. Comparison of the chemotactic responsiveness of two fibrosarcoma subpopulations of differing malignancy. Am J Pathol. 1981 Feb;102(2):160–167. [PMC free article] [PubMed] [Google Scholar]
- Peters B. P., Goldstein I. J. The use of fluorescein-conjugated Bandeiraea simplicifolia B4-isolectin as a histochemical reagent for the detection of alpha-D-galactopyranosyl groups. Their occurrence in basement membranes. Exp Cell Res. 1979 May;120(2):321–334. doi: 10.1016/0014-4827(79)90392-6. [DOI] [PubMed] [Google Scholar]
- Stanley W. S., Chu E. H. BS I-B4 isolectin as a probe for an investigation of membrane alterations and transformation phenotypes of mouse L cells. J Cell Sci. 1981 Aug;50:79–88. doi: 10.1242/jcs.50.1.79. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Liotta L. A., Russo R. G., Martin G. R. Role of laminin in the attachment and metastasis of murine tumor cells. Cancer Res. 1982 Jun;42(6):2265–2269. [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Varani J., Fantone J. C. Phorbol myristate acetate-induced adherence of Walker 256 carcinosarcoma cells. Cancer Res. 1982 Jan;42(1):190–197. [PubMed] [Google Scholar]
- Varani J., Lovett E. J., Elgebaly S., Lundy J., Ward P. A. In vitro and in vivo adherence of tumor cell variants correlated with tumor formation. Am J Pathol. 1980 Nov;101(2):345–352. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Lovett E. J. Phenotypic stability of murine tumor cells in vitro and in vivo. J Natl Cancer Inst. 1982 Jun;68(6):957–962. [PubMed] [Google Scholar]
- Varani J., Orr W., Ward P. A. Adhesive characteristics of tumor cell variants of high and low tumorigenic potential. J Natl Cancer Inst. 1980 May;64(5):1173–1178. [PubMed] [Google Scholar]
- Varani J., Orr W., Ward P. A. Comparison of subpopulations of tumor cells with altered migratory activity, attachment characteristics, enzyme levels and in vivo behavior. Eur J Cancer. 1979 Apr;15(4):585–592. doi: 10.1016/0014-2964(79)90096-3. [DOI] [PubMed] [Google Scholar]
- Varani J., Orr W., Ward P. A. Hydrolytic enzyme activities, migratory activity, and in vivo growth and metastatic potential of recent tumor isolates. Cancer Res. 1979 Jul;39(7 Pt 1):2376–2380. [PubMed] [Google Scholar]
- Wass J. A., Varani J., Piontek G. E., Ward P. A., Orr F. W. Responses of normal and malignant cells to collagen, collagen-derived peptides and the C5-related tumor cell chemotactic peptide. Cell Differ. 1981 Dec;10(6):329–332. doi: 10.1016/0045-6039(81)90024-5. [DOI] [PubMed] [Google Scholar]