Abstract
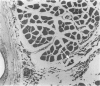
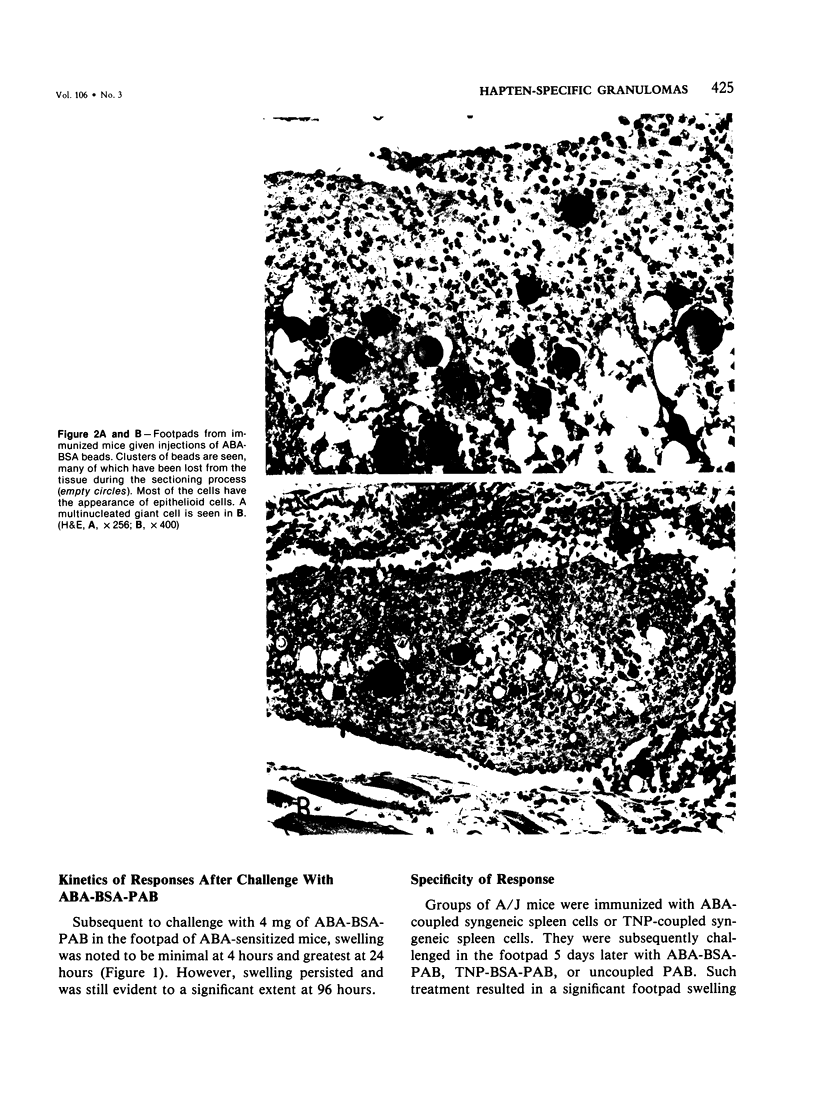
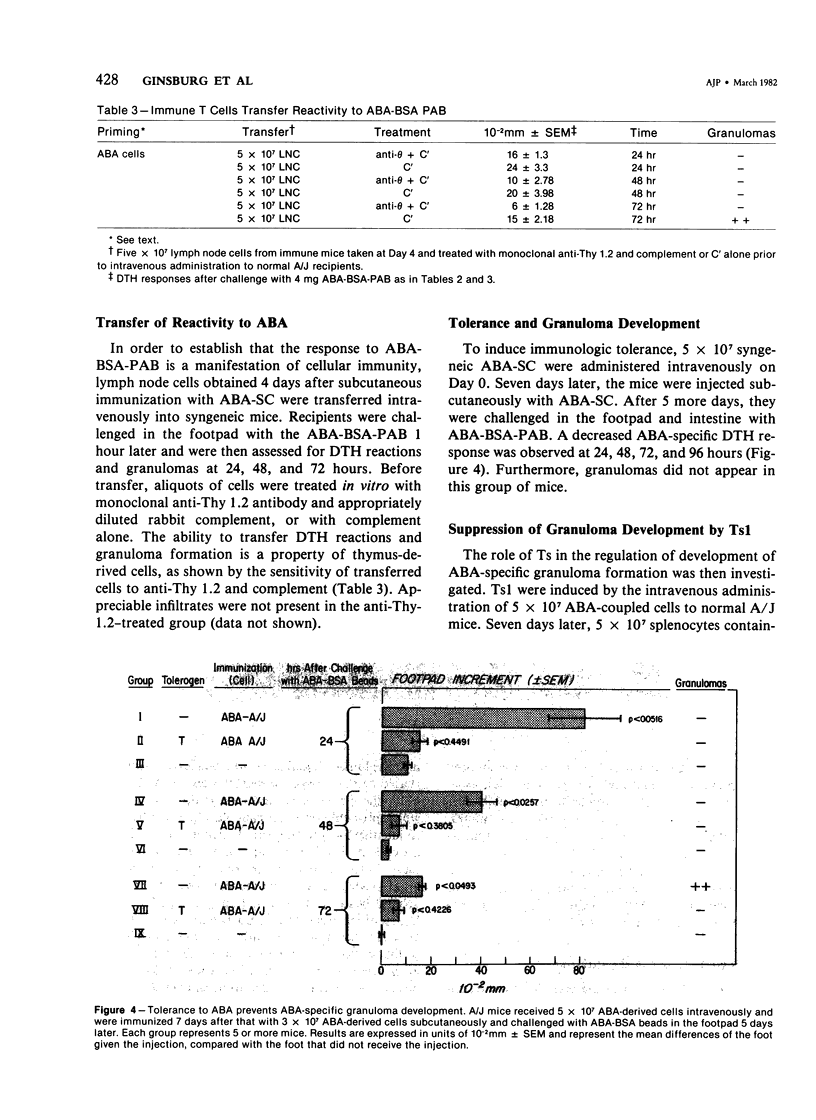
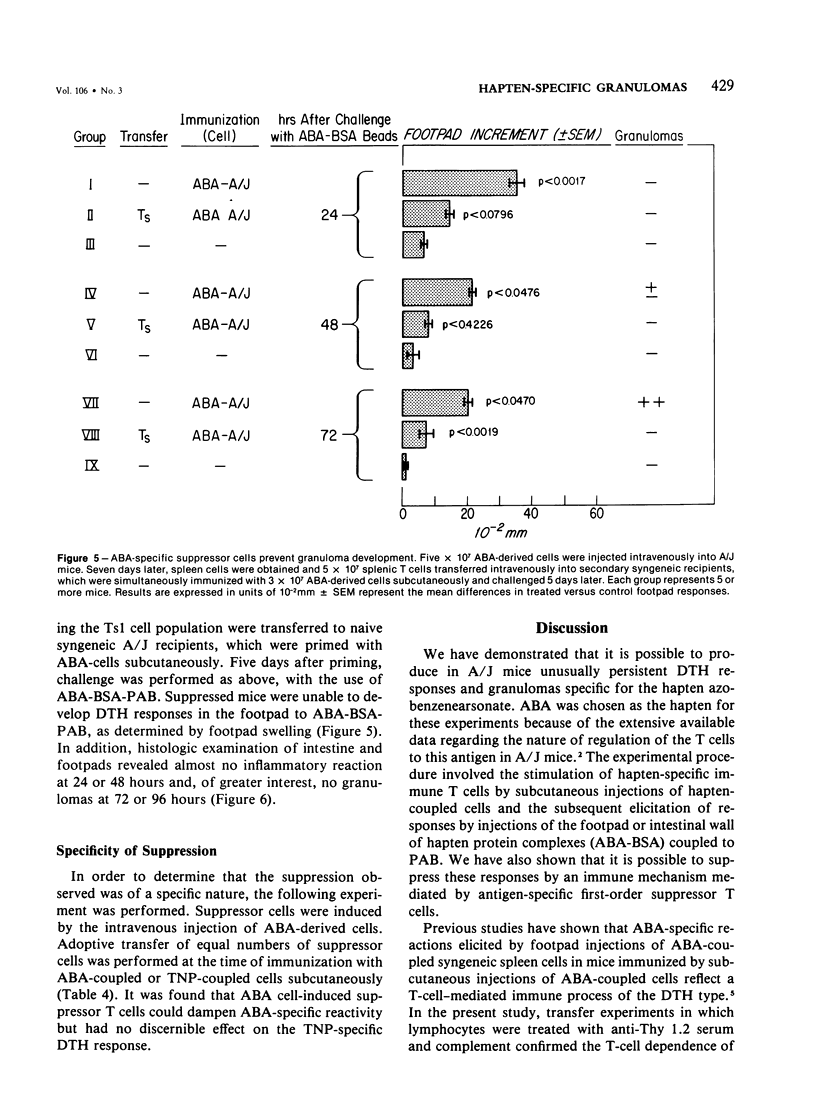
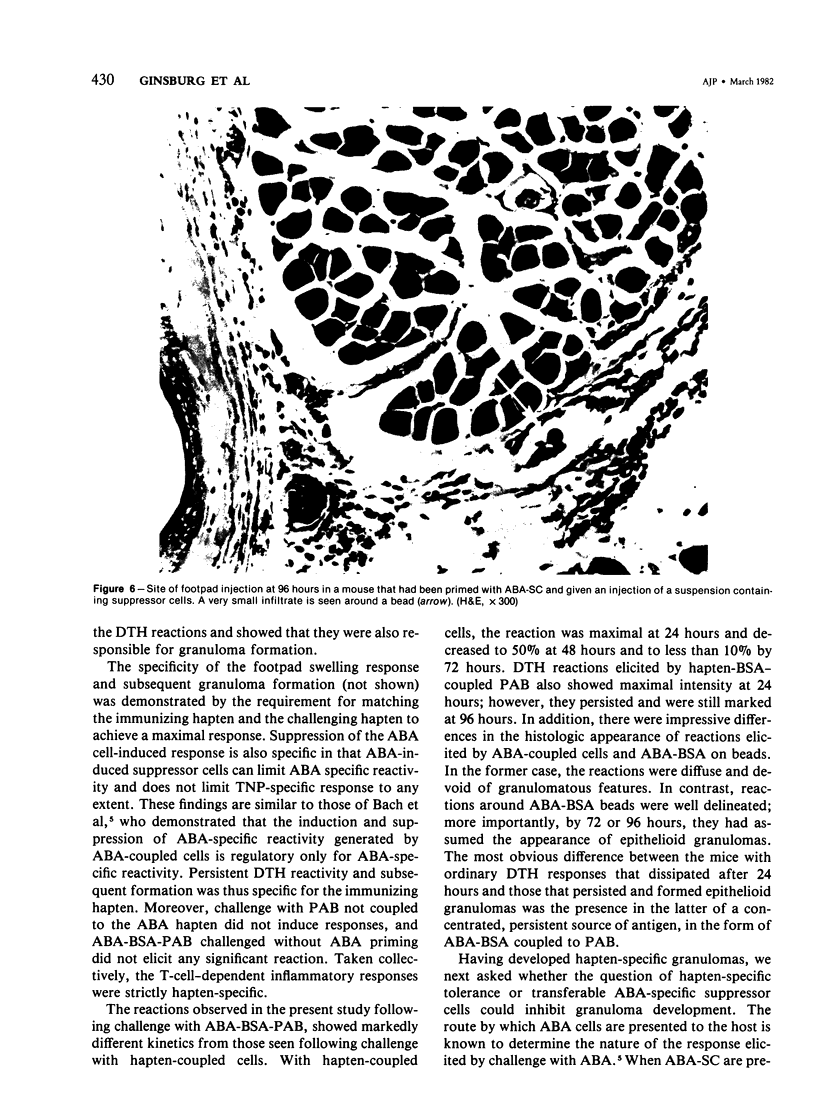
We have investigated the induction and suppression of granuloma formation elicited by the azobenzenearsonate (ABA) determinant in A/J Mice. ABA-derived syngeneic spleen cells (ABA-SC) administered subcutaneously induced hapten-specific delayed hypersensitivity (DTH), detected by footpad swelling, upon challenge with ABA-bovine serum albumin (BSA) coupled to polyacrylamide beads (PAB). The reactions elicited by ABA-BSA-PAB reached maximal intensity at 24 hours but were relatively persistent and were still marked at 96 hours. Histopathologic examination of footpad responses at 24 and 48 hours after challenge revealed compact collections around beads of mononuclear cells and granulocytes, which were characteristic of DTH reactions. Discrete epithelioid granulomas became apparent by 72 or 96 hours. Unprimed mice or mice primed with ABA-SC and challenged with uncoupled beads did not develop either substantial leukocytic infiltrates or granulomas. Persistent delayed responses were only apparent if the mice were challenged with the homologous hapten-coupled bead, indicating the fine specificity of the reaction. Immune cells were shown to be capable of transferring DTH and granulomatous responsiveness to ABA; the cells were sensitive to anti-Thy 1.2 antiserum and complement, which indicates that the response was thymic-dependent. The intravenous injection of ABA-SC, which is known to induce suppressor cells, prevented the development of DTH or granulomatous responsiveness followinggg subcutaneous immunization with ABA-SC. In addition, the transfer of suspensions containing suppressor T cells into syngeneic mice primed with ABA-SC prevented the development of DTH reactions and granuloma formation followin challenge with ABA-BSA-PAB. Furthermore, only hapten-specific suppressor T cells limited persistent delayed hypersensitivity responses. Having successfully developed granulomas in the footpad, the authors induced and suppressed granulomatous lesions in the gastrointestinal tract in a similar fashion. These experiments establish a model in inbred mice for the study of granulomatous diseases, including those of the gastrointestinal tract.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach B. A., Sherman L., Benacerraf B., Greene M. I. Mechanisms of regulation of cell-mediated immunity. II. Induction and suppression of delayed-type hypersensitivity to azobenzenearsonate-coupled syngeneic cells. J Immunol. 1978 Oct;121(4):1460–1468. [PubMed] [Google Scholar]
- Claman H. N., Miller S. D., Sy M. S., Moorhead J. W. Suppressive mechanisms involving sensitization and tolerance in contact allergy. Immunol Rev. 1980;50:105–132. doi: 10.1111/j.1600-065x.1980.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Colley D. G. T lymphocytes that contribute to the immunoregulation of granuloma formation in chronic murine schistosomiasis. J Immunol. 1981 Apr;126(4):1465–1468. [PubMed] [Google Scholar]
- Greene M. I., Bach B. A., Benacerraf B. Mechanisms of regulation of cell-mediated immunity. III. The characterization of azobenzenearsonate-specific suppressor T-cell-derived-suppressor factors. J Exp Med. 1979 May 1;149(5):1069–1083. doi: 10.1084/jem.149.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak L. L., Mushinski E. B., Nisonoff A., Potter M. Evidence for the linkage of the IGC H locus to a gene controlling the idiotypic specificity of anti-p-azophenylarsonate antibodies in strain A mice. J Exp Med. 1973 Jan 1;137(1):22–31. doi: 10.1084/jem.137.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy M. S., Bach B. A., Brown A., Nisonoff A., Benacerraf B., Greene M. I. Antigen- and receptor-driven regulatory mechanisms. II. Induction of suppressor T cells with idiotype-coupled syngeneic spleen cells. J Exp Med. 1979 Nov 1;150(5):1229–1240. doi: 10.1084/jem.150.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy M. S., Brown A., Bach B. A., Benacerraf B., Gottlieb P. D., Nisonoff A., Greene M. I. Genetic and serological analysis of the expression of crossreactive idiotypic determinants on anti-p-azobenzenarsonate antibodies and p-azobenzenarsonate-specific suppressor T cell factors. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1143–1147. doi: 10.1073/pnas.78.2.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy M. S., Dietz M. H., Germain R. N., Benacerraf B., Greene M. I. Antigen- and receptor-driven regulatory mechanisms. IV. Idiotype-bearing I-J+ suppressor T cell factors induce second-order suppressor T cells which express anti-idiotypic receptors. J Exp Med. 1980 May 1;151(5):1183–1195. doi: 10.1084/jem.151.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy M. S., Nisonoff A., Germain R. N., Benacerraf B., Greene M. I. Antigen- and receptor-driven regulatory mechanisms. VIII. Suppression of idiotype-negative, p-azobenzenearsonate-specific T cells results from the interaction of an anti-idiotypic second-order T suppressor cell with a cross-reactive-idiotype-positive, p-azobenzenearsonate-primed T cell target. J Exp Med. 1981 Jun 1;153(6):1415–1425. doi: 10.1084/jem.153.6.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Benacerraf B. Immunologic events in experimental hypersensitivity granulomas. Am J Pathol. 1973 Jun;71(3):349–364. [PMC free article] [PubMed] [Google Scholar]