Abstract
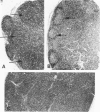
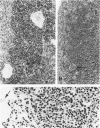
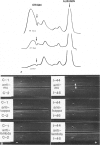
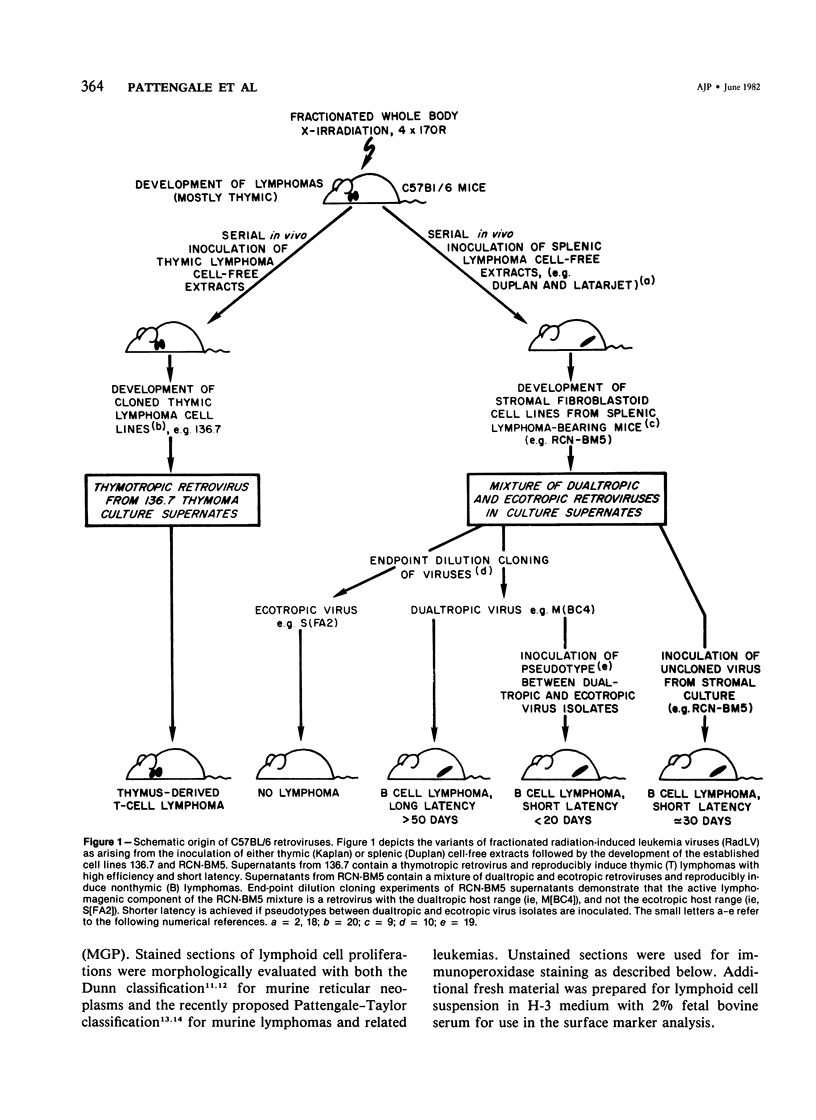
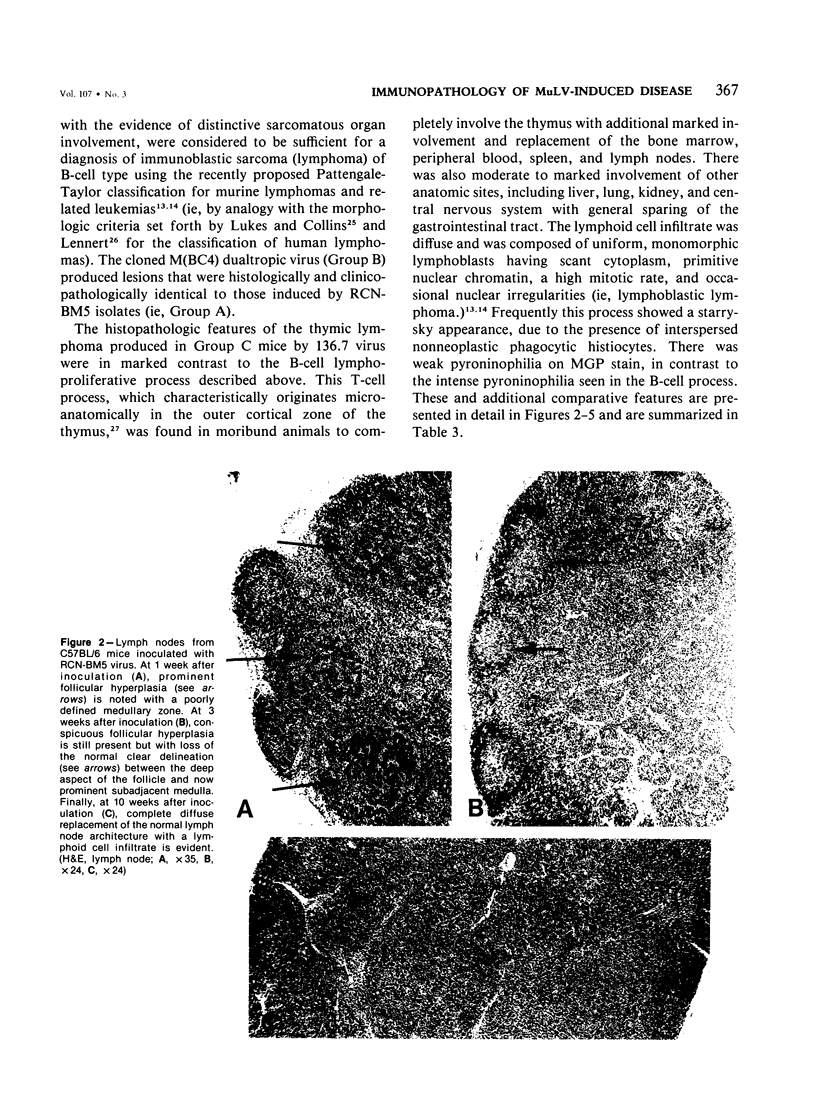
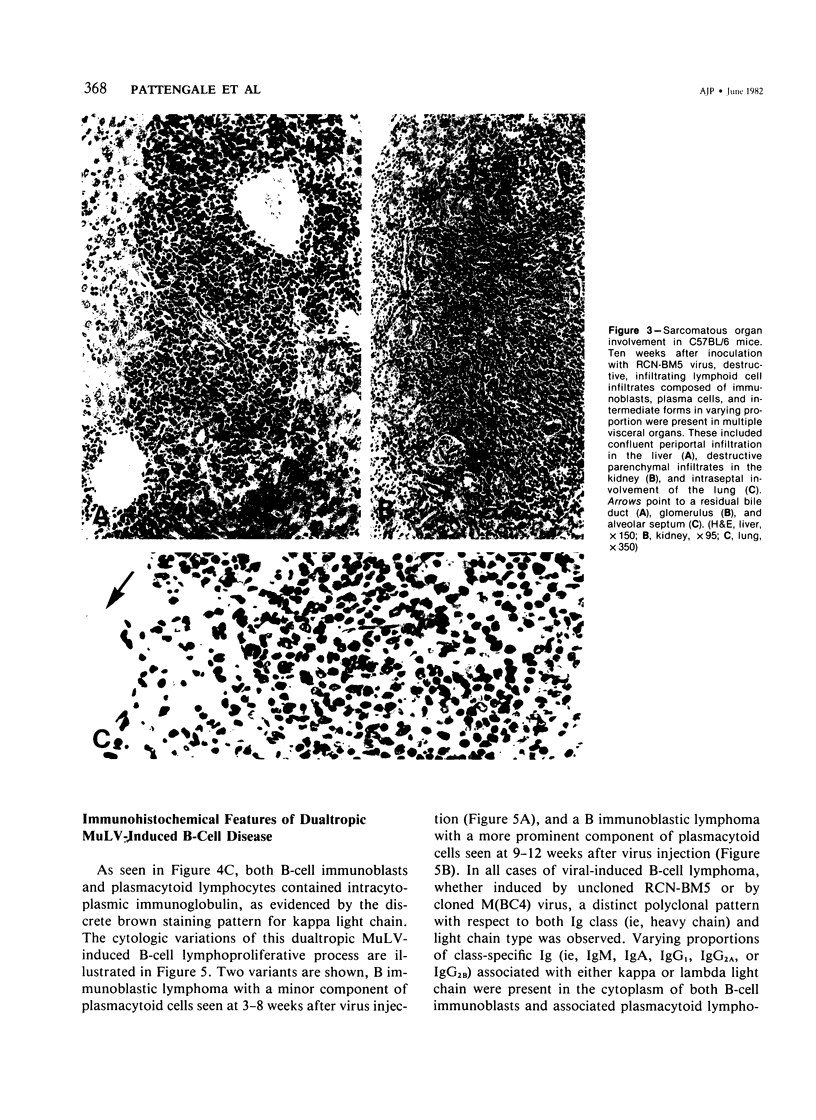
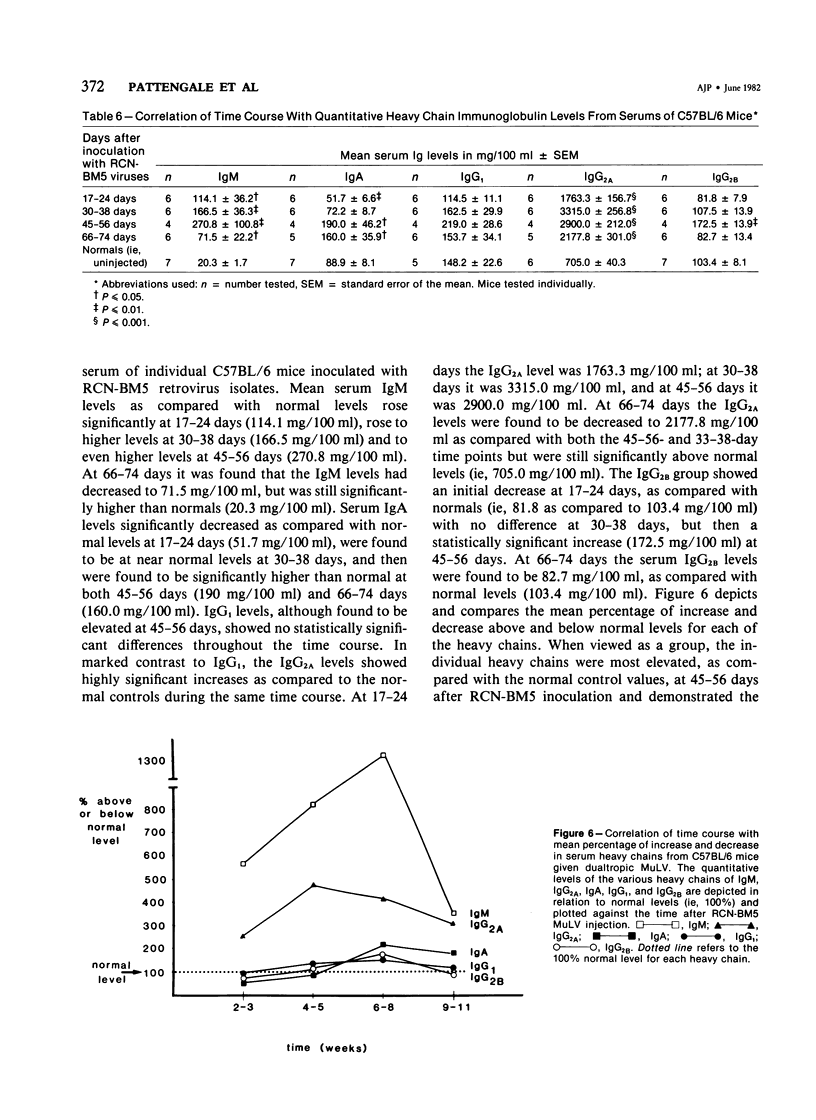
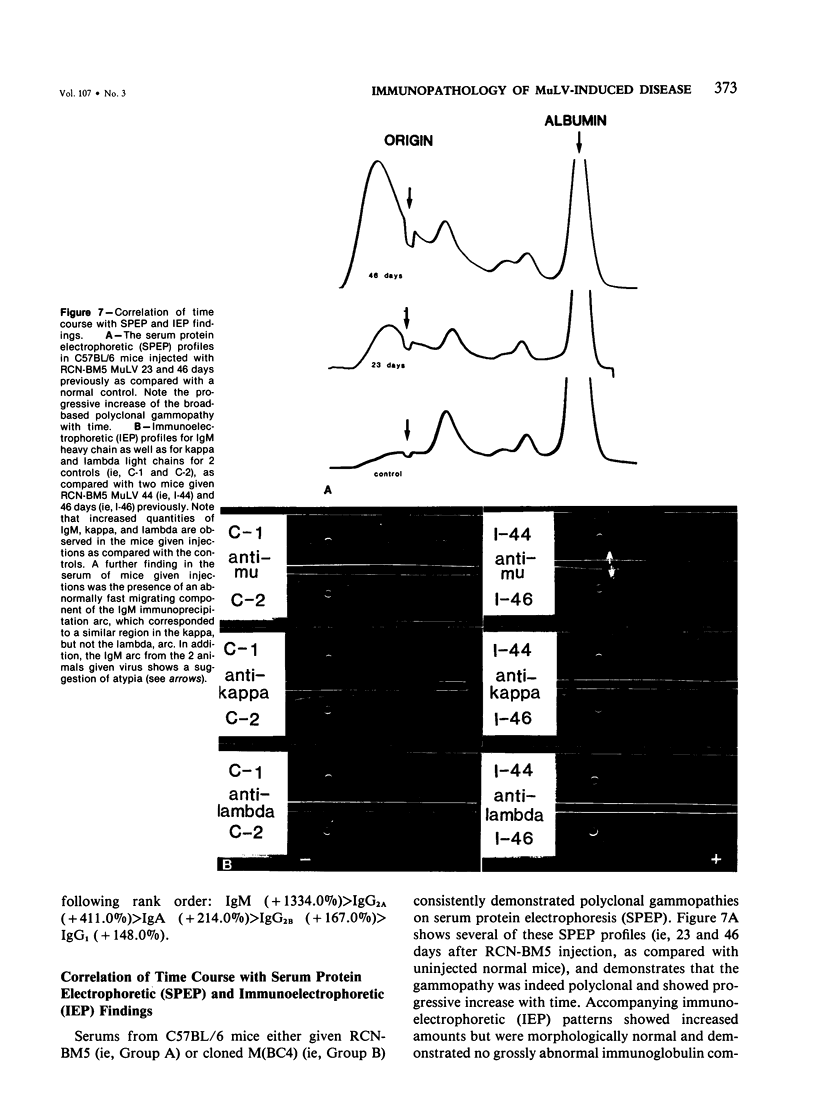
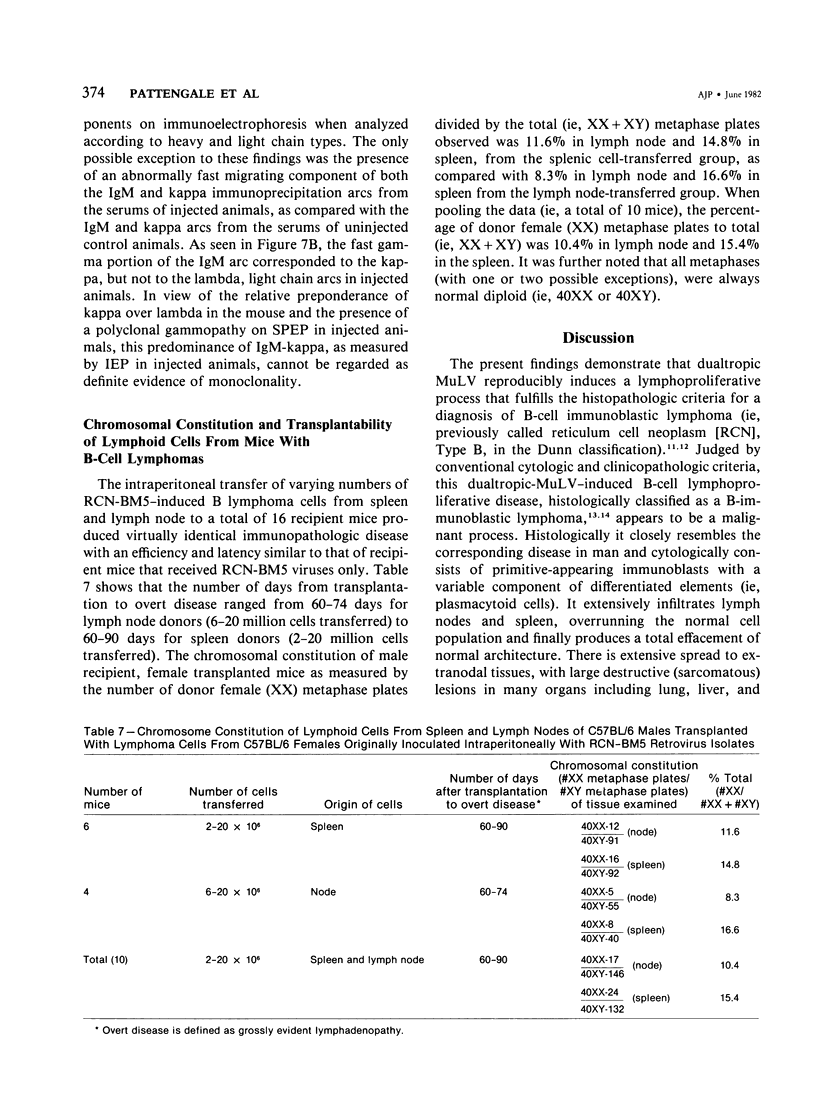
Combined clinicopathologic and immunomorphologic evidence is presented that would indicate that a murine leukemia virus (MuLV) with the dualtropic host range is capable of producing a clinically malignant lesion composed of immunoblasts and associated plasma cells in C57BL/6 mice. This process, morphologically diagnosed as an immunoblastic lymphoma of B cells using standard histopathologic criteria, was found to be distinctly polyclonal with regard to immunoglobulin (Ig) isotype when analyzed for both surface and cytoplasmic Ig. Further studies demonstrated that this clinicopathologically malignant, dualtropic MuLV-induced, polyclonal immunoblastic lymphoma of B cells in C57BL/6 mice was normal diploid and unable to be successfully transplanted to nonimmunosuppressed syngeneic recipients. Although all serum heavy and light chain components were found to be progressively elevated as the tumor load increased, the polyclonal increase in serum immunoglobulins was most pronounced for mu heavy and kappa light chains (ie, mu greater than gamma 2A greater than alpha greater than gamma 2B greater than gamma 1; kappa greater than lamba). The dissociation of clinicopathologic and biologic criteria for malignancy in the presently described dualtropic (RadLV) MuLV-induced B-cell lesion is sharply contrasted with the thymotropic (RadLV), MuLV-induced T-cell lymphoblastic lymphoma in C57BL/6 mice. This process is also a clinicopathologically malignant lesion but, when one uses biologic criteria, is found to be distinctly monoclonal, aneuploid, and easily transplanted to nonimmunosuppressed syngeneic recipients. The close clinicopathologic and biologic similarities of the dualtropic MuLV-induced animal model to corresponding human B-cell lymphoproliferative diseases are stressed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caspersson T., Farber S., Foley G. E., Kudynowski J., Modest E. J., Simonsson E., Wagh U., Zech L. Chemical differentiation along metaphase chromosomes. Exp Cell Res. 1968 Jan;49(1):219–222. doi: 10.1016/0014-4827(68)90538-7. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNN T. B. Normal and pathologic anatomy of the reticular tissue in laboratory mice, with a classification and discussion of neoplasms. J Natl Cancer Inst. 1954 Jun;14(6):1281–1433. [PubMed] [Google Scholar]
- Duplan J. F., Latarjet R. Studies on the mechanism of radiation-induced leukemogenesis in C57BL mice. Cancer Res. 1966 Mar;26(3):395–399. [PubMed] [Google Scholar]
- Famulari N. G., Jelalian K. Cell surface expression of the env gene polyprotein of dual-tropic mink cell focus-forming murine leukemia virus. J Virol. 1979 Jun;30(3):720–728. doi: 10.1128/jvi.30.3.720-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulari N. G., Tung J. S., O'Donnell P. V., Fleissner E. Murine leukemia virus env-gene expression in preleukemic thymocytes and leukemia cells of AKR strain mice. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1281–1287. doi: 10.1101/sqb.1980.044.01.140. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Nomura S., Bolognesi D. P. A novel murine oncornavirus with dual eco- and xenotropic properties. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5150–5155. doi: 10.1073/pnas.72.12.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., Meshorer A. Reticulum cell neoplasms induced in C57BL/6 mice by cultured virus grown in stromal hematopoietic cell lines. J Natl Cancer Inst. 1979 Aug;63(2):427–439. [PubMed] [Google Scholar]
- Haas M., Patch V. Cell-surface antigens associated with dualtropic and thymotropic murine leukemia viruses inducing thymic and nonthymic lymphomas. J Exp Med. 1980 Jun 1;151(6):1321–1333. doi: 10.1084/jem.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., Patch V. Genomic masking and rescue of dual-tropic murine leukemia viruses: role of pseudotype virions in viral lymphomagenesis. J Virol. 1980 Sep;35(3):583–591. doi: 10.1128/jvi.35.3.583-591.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., Reshef T. Non-thymic malignant lymphomas induced in C57BL/6 mice by cloned dualtropic viruses isolated from hematopoietic stromal cell lines. Eur J Cancer. 1980 Jul;16(7):909–917. doi: 10.1016/0014-2964(80)90329-1. [DOI] [PubMed] [Google Scholar]
- Haran-Ghera N. Leukemogenic activity of centrifugates from irradiated mouse thymus and bone marrow. Int J Cancer. 1966 Jan;1(1):81–87. doi: 10.1002/ijc.2910010111. [DOI] [PubMed] [Google Scholar]
- Haran-Ghera N., Lieberman M., Kaplan H. S. Direct action of a leukemogenic virus on the thymus. Cancer Res. 1966 Mar;26(3):438–442. [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN H. S. Preliminary studies of the effectiveness of local irradiation in the induction of lymphoid tumors in mice. J Natl Cancer Inst. 1949 Oct;10(2):267–270. [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- Klein G. Lymphoma development in mice and humans: diversity of initiation is followed by convergent cytogenetic evolution. Proc Natl Acad Sci U S A. 1979 May;76(5):2442–2446. doi: 10.1073/pnas.76.5.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Purtilo D. Summary: symposium on Epstein-Barr virus-induced lymphoproliferative diseases in immunodeficient patients. Cancer Res. 1981 Nov;41(11 Pt 1):4302–4304. [PubMed] [Google Scholar]
- LATARJET R., DUPLAN J. F. Experiment and discussion on leukaemogenesis by cell-free extracts of radiation-induced leukaemia in mice. Int J Radiat Biol Relat Stud Phys Chem Med. 1962 Aug;5:339–344. doi: 10.1080/09553006214550911. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN M., KAPLAN H. S. Leukemogenic activity of filtrates from radiation-induced lymphoid tumors of mice. Science. 1959 Aug 14;130(3372):387–388. doi: 10.1126/science.130.3372.387. [DOI] [PubMed] [Google Scholar]
- Lennert K., Stein H., Kaiserling E. Cytological and functional criteria for the classification of malignant lymphomata. Br J Cancer Suppl. 1975 Mar;2:29–43. [PMC free article] [PubMed] [Google Scholar]
- Lukes R. J., Collins R. D. New approaches to the classification of the lymphomata. Br J Cancer Suppl. 1975 Mar;2:1–28. [PMC free article] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Giovanella B. C., Stehlin J. S., Klein G. Tumorigenicity of human hematopoietic cell lines in athymic nude mice. Int J Cancer. 1977 Mar 15;19(3):337–344. doi: 10.1002/ijc.2910190309. [DOI] [PubMed] [Google Scholar]
- Pattengale P. K., Smith R. W., Gerber P. B-cell characteristics of human peripheral and cord blood lymphocytes transformed by Epstein-Barr virus. J Natl Cancer Inst. 1974 Apr;52(4):1081–1086. doi: 10.1093/jnci/52.4.1081. [DOI] [PubMed] [Google Scholar]
- Pattengale P. K., Smith R. W., Gerber P. Selective transformation of B lymphocytes by E.B. virus. Lancet. 1973 Jul 14;2(7820):93–94. doi: 10.1016/s0140-6736(73)93286-8. [DOI] [PubMed] [Google Scholar]
- Pattengale P. K., Taylor C. R., Panke T., Tatter D., McCormick R. A., Rawlinson D. G., Davis R. L. Selective immunodeficiency and malignant lymphoma of the central nervous system. Possible relationship to the Epstein-Barr virus. Acta Neuropathol. 1979 Dec;48(3):165–169. doi: 10.1007/BF00690516. [DOI] [PubMed] [Google Scholar]
- Pattengale P. K., Taylor C. R., Pegalow C. Malignant B-cell lymphoma following and associated with infectious mononucleosis. A comparison of two cases. Am J Pediatr Hematol Oncol. 1981 Spring;3(1):35–42. [PubMed] [Google Scholar]
- Penn I. Occurrence of cancer in immune deficiencies. Cancer. 1974 Sep;34(3):suppl–suppl:866. doi: 10.1002/1097-0142(197409)34:3+<858::aid-cncr2820340712>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T., DeFlorio D., Jr, Hutt L. M., Bhawan J., Yang J. P., Otto R., Edwards W. Variable phenotypic expression of an X-linked recessive lymphoproliferative syndrome. N Engl J Med. 1977 Nov 17;297(20):1077–1080. doi: 10.1056/NEJM197711172972001. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T. Pathogenesis and phenotypes of an X-linked recessive lymphoproliferative syndrome. Lancet. 1976 Oct 23;2(7991):882–885. doi: 10.1016/s0140-6736(76)90542-0. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T., Yang J. P., Allegra S., DeFlorio D., Hutt L. M., Soltani M., Soltani M., Vawter G. Hematopathology and Pathogenesis of the X-linked recessive lymphoproliferative syndrome. Am J Med. 1977 Feb;62(2):225–233. doi: 10.1016/0002-9343(77)90318-7. [DOI] [PubMed] [Google Scholar]
- ROTHFELS K. H., SIMINOVITCH L. An air-drying technique for flattening chromosomes in mammalian oells grown in vitro. Stain Technol. 1958 Mar;33(2):73–77. doi: 10.3109/10520295809111827. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L., Byron K. S., Brewster F. E., Purtilo D. T. Deficient natural killer cell activity in x-linked lymphoproliferative syndrome. Science. 1980 Oct 31;210(4469):543–545. doi: 10.1126/science.6158759. [DOI] [PubMed] [Google Scholar]
- Taylor C. R. Immunoperoxidase techniques: practical and theoretical aspects. Arch Pathol Lab Med. 1978 Mar;102(3):113–121. [PubMed] [Google Scholar]
- van Griensven L. J., Vogt M. Rauscher "mink cell focus-inducing" (MCF) virus causes erythroleukemia in mice: its isolation and properties. Virology. 1980 Mar;101(2):376–388. doi: 10.1016/0042-6822(80)90451-1. [DOI] [PubMed] [Google Scholar]