Abstract
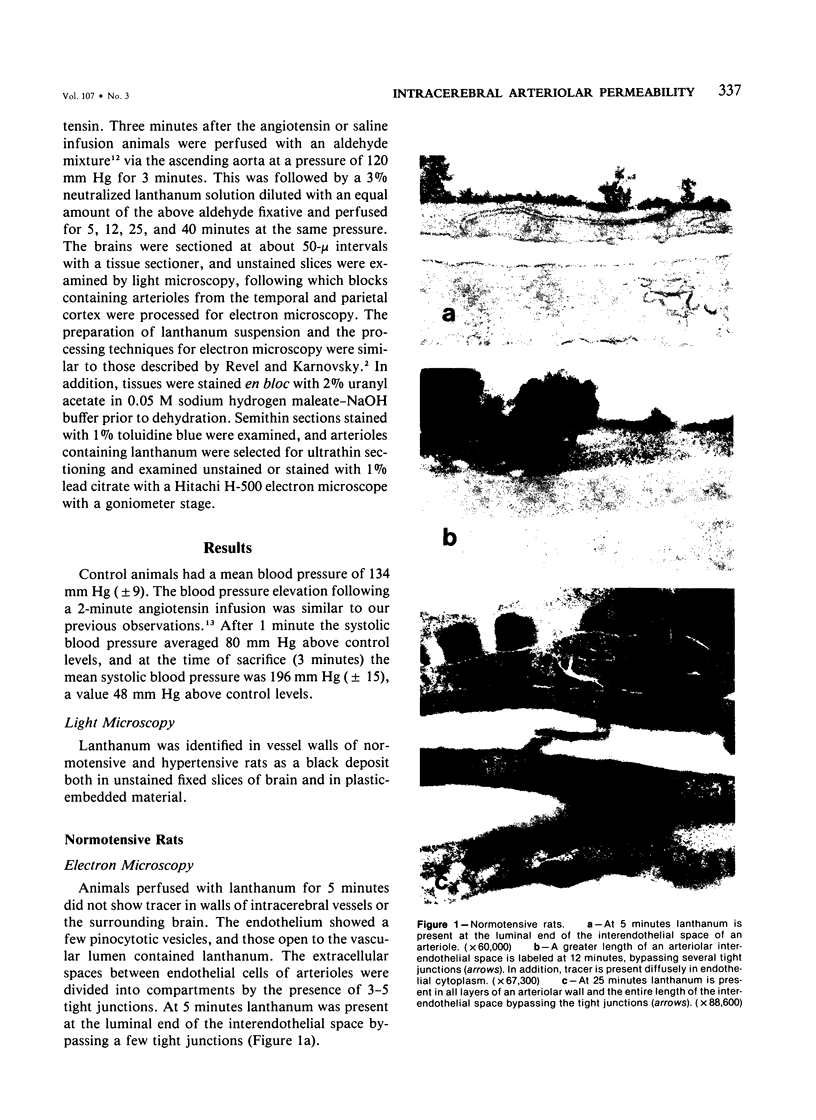
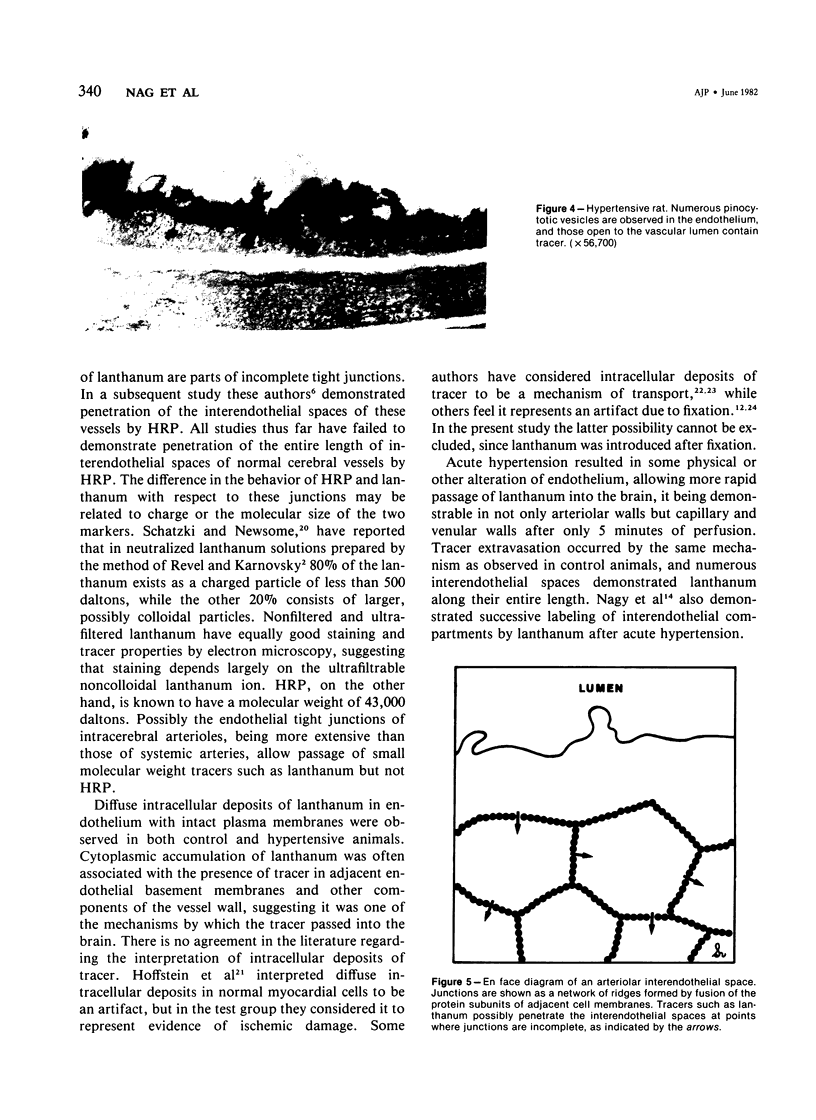
Lanthanum, an electron-dense tracer, has been used extensively in the study of the structure of cell junctions. The present study was undertaken to determine whether the interendothelial junctions of normal intracerebral arterioles allow passage of lanthanum and to document the alterations occurring in these structures in acute hypertension. Perfusion of lanthanum for 12-40 minutes in control animals resulted in passage of tracer into arteriolar walls and into the extracellular compartment of the surrounding brain. The two principal mechanisms associated with tracer extravasation into the brain were diffuse passage through endothelial cytoplasm and through interendothelial spaces bypassing tight junctions. The latter finding has not been previously reported in normal cerebral arterioles and suggests that the tight junctions of these vessels are different from those of capillaries and consist of a meshwork of closely arranged maculae occludentes rather than complete circumferential occluding bands as was previously believed. Hypertensive animals showed accelerated passage of lanthanum, it being demonstrable not only in arteriolar walls but in capillary and venular walls and the surrounding neuropil after only 5 minutes of circulation. Passage of tracer through vessel walls occurred by the same routes as in control. In addition, increased numbers of pinocytotic vesicles were observed in the endothelium, confirming our previous studies that increased vesicular transport occurs in cerebral arteriolar endothelium in acute hypertension.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouldin T. W., Krigman M. R. Differential permeability of cerebral capillary and choroid plexus to lanthanum ion. Brain Res. 1975 Dec 5;99(2):444–448. doi: 10.1016/0006-8993(75)90053-0. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Klatzo I., Olsson Y., Reese T. S. The blood-brain barrier to proteins under normal and pathological conditions. J Neurol Sci. 1970 Mar;10(3):215–239. doi: 10.1016/0022-510x(70)90151-6. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell C. J., Mercer K. L. Freeze-fracture appearance of the capillary endothelium in the cerebral cortex of mouse brain. Am J Anat. 1974 Aug;140(4):595–599. doi: 10.1002/aja.1001400412. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A., Revel J. P. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol. 1970 May;45(2):272–290. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A., Becker N. H., Zimmerman H. M. Pathological alterations in the cerebral endothelial cell barrier to peroxidase. Arch Neurol. 1969 Mar;20(3):300–308. doi: 10.1001/archneur.1969.00480090088013. [DOI] [PubMed] [Google Scholar]
- Hoffstein S., Gennaro D. E., Fox A. C., Hirsch J., Streuli F., Weissmann G. Colloidal lanthanum as a marker for impaired plasma membrane permeability in ischemic dog myocardium. Am J Pathol. 1975 May;79(2):207–218. [PMC free article] [PubMed] [Google Scholar]
- Hüttner I., Boutet M., More R. H. Studies on protein passage through arterial endothelium. I. Structural correlates of permeability in rat arterial endothelium. Lab Invest. 1973 Jun;28(6):672–677. [PubMed] [Google Scholar]
- Hüttner I., Boutet M., More R. H. Studies on protein passage through arterial endothelium. II. Regional differences in permeability to fine structural protein tracers in arterial endothelium of normotensive rat. Lab Invest. 1973 Jun;28(6):678–685. [PubMed] [Google Scholar]
- Karnovsky M. J. The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol. 1967 Oct;35(1):213–236. doi: 10.1083/jcb.35.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt N. S., Weinstein R. S. The ultrastructure of the nexus. A correlated thin-section and freeze-cleave study. J Cell Biol. 1970 Dec;47(3):666–688. doi: 10.1083/jcb.47.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollgøard K., Saunders N. R. Complex tight junctions of epithelial and of endothelial cells in early foetal brain. J Neurocytol. 1975 Aug;4(4):453–468. doi: 10.1007/BF01261375. [DOI] [PubMed] [Google Scholar]
- Nag S., Robertson D. M., Dinsdale H. B. Cerebral cortical changes in acute experimental hypertension: An ultrastructural study. Lab Invest. 1977 Feb;36(2):150–161. [PubMed] [Google Scholar]
- Nag S., Robertson D. M., Dinsdale H. B. Morphological changes in spontaneously hypertensive rats. Acta Neuropathol. 1980;52(1):27–34. doi: 10.1007/BF00687225. [DOI] [PubMed] [Google Scholar]
- Nag S., Robertson D. M., Dinsdale H. B. Quantitative estimate of pinocytosis in experimental acute hypertension. Acta Neuropathol. 1979 Apr 12;46(1-2):107–116. doi: 10.1007/BF00684811. [DOI] [PubMed] [Google Scholar]
- Nagy Z., Mathieson G., Hüttner I. Blood-brain barrier opening to horseradish peroxidase in acute arterial hypertension. Acta Neuropathol. 1979 Oct;48(1):45–53. doi: 10.1007/BF00691790. [DOI] [PubMed] [Google Scholar]
- Petito C. K., Levy D. E. The importance of cerebral arterioles in alterations of the blood-brain barrier. Lab Invest. 1980 Sep;43(3):262–268. [PubMed] [Google Scholar]
- Reese T. S., Karnovsky M. J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967 Jul;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel J. P., Karnovsky M. J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967 Jun;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzki P. F., Newsome A. Neutralized lanthanum solution: a largely noncolloidal ultrastructural tracer. Stain Technol. 1975 May;50(3):171–178. doi: 10.3109/10520297509117054. [DOI] [PubMed] [Google Scholar]
- Westergaard E. The blood-brain barrier to horseradish peroxidase under normal and experimental conditions. Acta Neuropathol. 1977 Aug 31;39(3):181–187. doi: 10.1007/BF00691695. [DOI] [PubMed] [Google Scholar]






