Full text
PDF


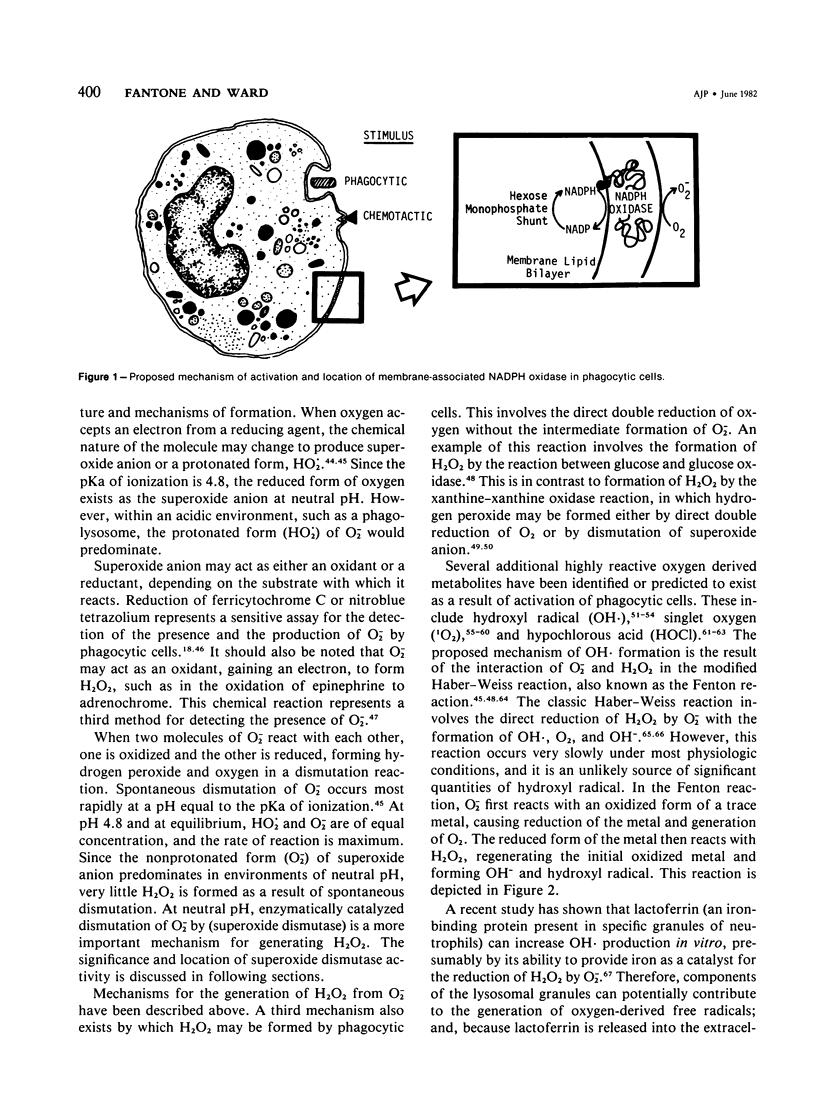

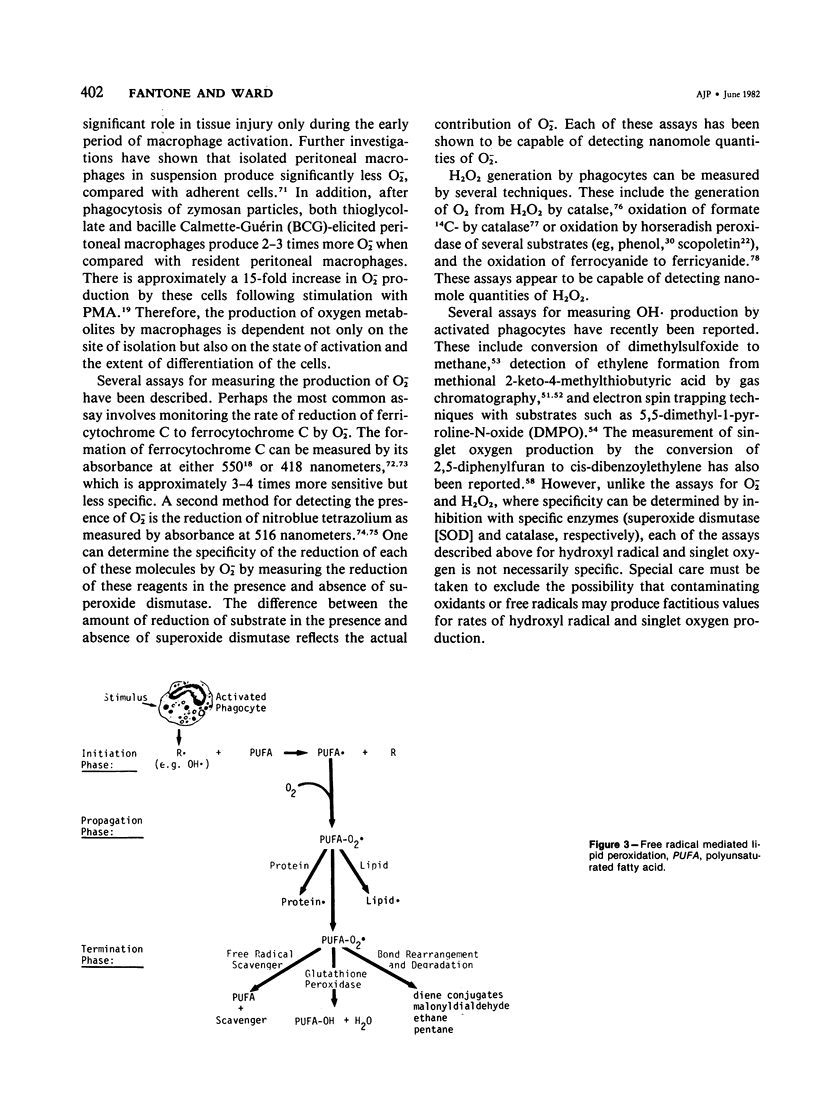
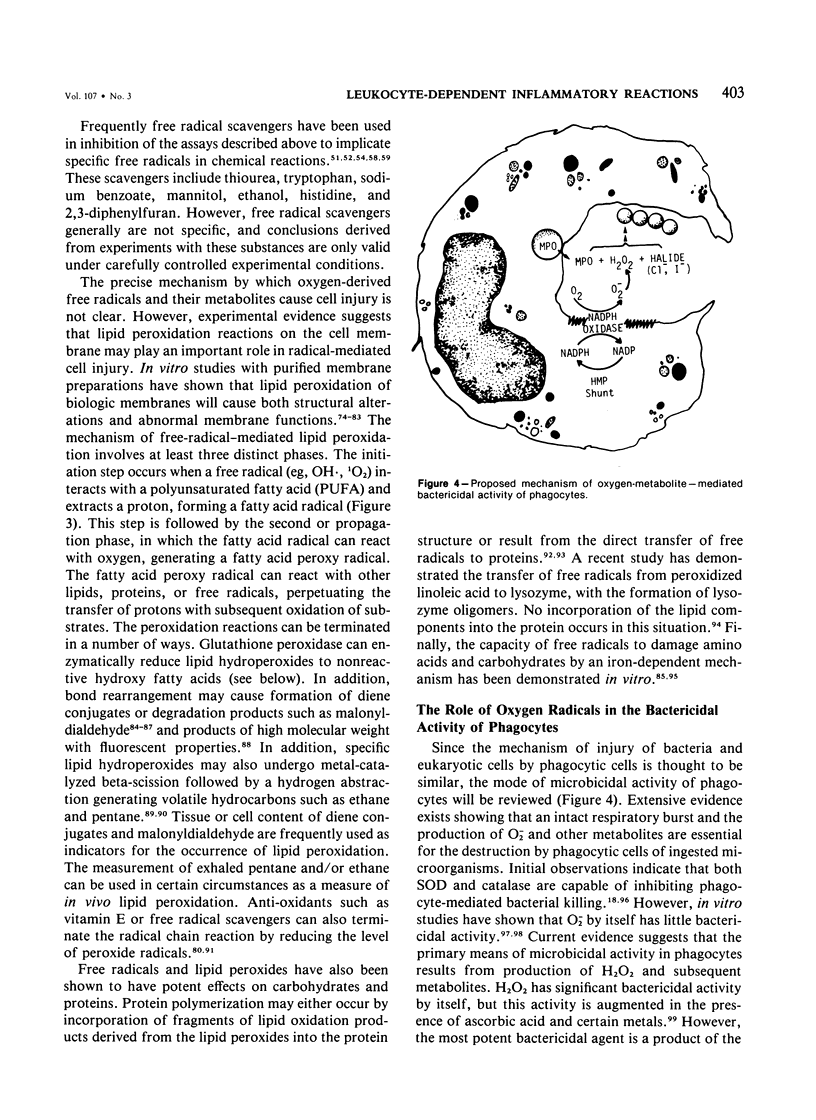



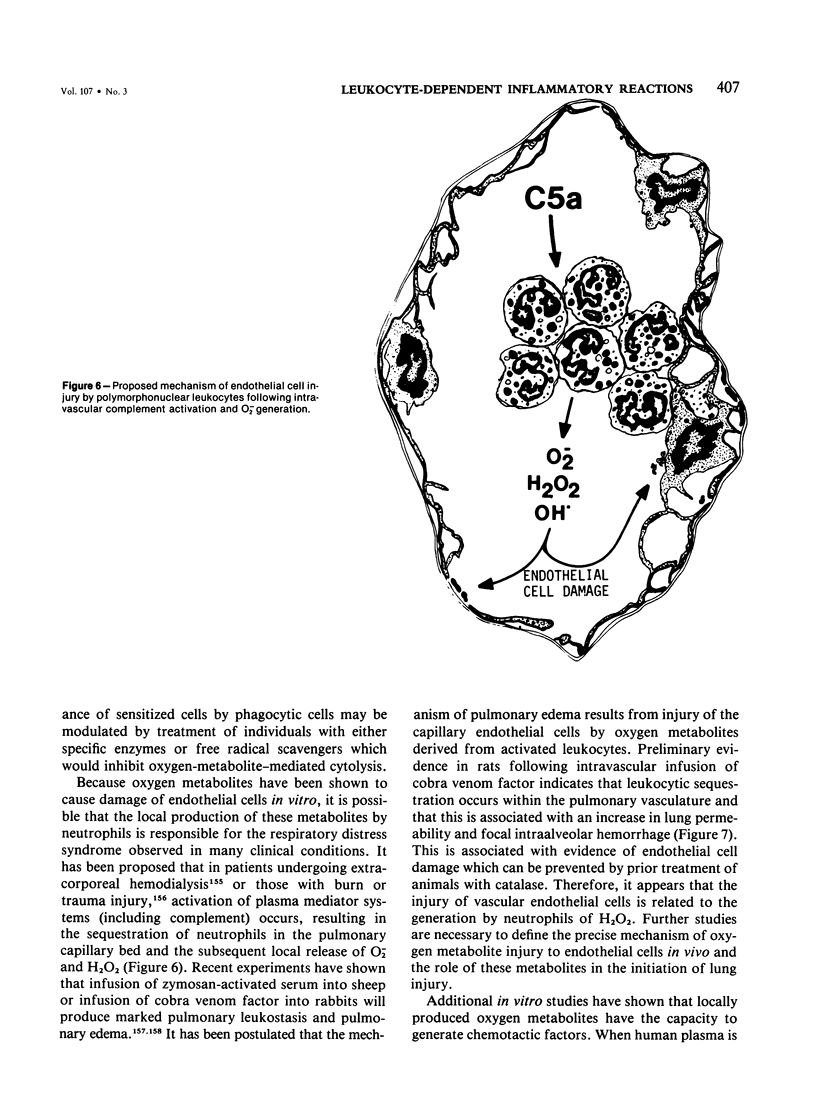










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Timimi D. J., Dormandy T. L. The inhibition of lipid autoxidation by human caeruloplasmin. Biochem J. 1977 Nov 15;168(2):283–288. doi: 10.1042/bj1680283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Ambruso D. R., Johnston R. B., Jr Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J Clin Invest. 1981 Feb;67(2):352–360. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen B. R., Brendzel A. M., Lint T. F. Chemiluminescence spectra of human myeloperoxidase and polymorphonuclear leukocytes. Infect Immun. 1977 Jul;17(1):62–66. doi: 10.1128/iai.17.1.62-66.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARBER A. A. Inhibition of lipid peroxide formation by vertebrate blood serum. Arch Biochem Biophys. 1961 Jan;92:38–43. doi: 10.1016/0003-9861(61)90215-6. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., McMurrich B. J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976 Oct;58(4):989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Babior G. L., Rosin R. E., McMurrich B. J., Peters W. A., Babior B. M. Arrangement of the respiratory burst oxidase in the plasma membrane of the neutrophil. J Clin Invest. 1981 Jun;67(6):1724–1728. doi: 10.1172/JCI110210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Allen J. M., Davis J. Autooxidation as a basis for altered function by polymorphonuclear leukocytes. Blood. 1977 Aug;50(2):327–335. [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Davis J. The biochemical basis of nitroblue tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood. 1976 Aug;48(2):309–313. [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Davis J. The biochemical basis of nitroblue tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood. 1976 Aug;48(2):309–313. [PubMed] [Google Scholar]
- Baehner R. L., Johnston R. B., Jr, Nathan D. G. Comparative study of the metabolic and bactericidal characteristics of severely glucose-6-phosphate dehydrogenase-deficient polymorphonuclear leukocytes and leukocytes from children with chronic granulomatous disease. J Reticuloendothel Soc. 1972 Aug;12(2):150–169. [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H., Hill H. A., Mahood J. F., Willson R. L., Wolfenden B. S. Does caeruloplasmin dismute superoxide? No. FEBS Lett. 1980 Aug 25;118(1):127–129. doi: 10.1016/0014-5793(80)81233-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Sigman M., Oliver J. M. Superoxide production induced in rabbit polymorphonuclear leukocytes by synthetic chemotactic peptides and A23187. Am J Pathol. 1979 Apr;95(1):81–97. [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Ismail G., Allen J. M., Baehner R. L. Oxidative metabolic responses of rabbit pulmonary alveolar macrophages. Blood. 1979 Mar;53(3):486–491. [PubMed] [Google Scholar]
- CSALLANY A. S., DRAPER H. H. Determination of N,N'-diphenyl-p-phenylenediamine in animal tissues. Proc Soc Exp Biol Med. 1960 Aug-Sep;104:739–742. doi: 10.3181/00379727-104-25971. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson S., Vogin E. E., Huber W., Schulte T. L. Safety tests of orgotein, an antiinflammatory protein. Toxicol Appl Pharmacol. 1973 Oct;26(2):184–202. doi: 10.1016/0041-008x(73)90252-4. [DOI] [PubMed] [Google Scholar]
- Cheson B. D., Christensen R. L., Sperling R., Kohler B. E., Babior B. M. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976 Oct;58(4):789–796. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C. K. Distribution of tocopherols in human plasma and red blood cells. Am J Clin Nutr. 1975 Jul;28(7):756–760. doi: 10.1093/ajcn/28.7.756. [DOI] [PubMed] [Google Scholar]
- Chow C. K. Nutritional influence on cellular antioxidant defense systems. Am J Clin Nutr. 1979 May;32(5):1066–1081. doi: 10.1093/ajcn/32.5.1066. [DOI] [PubMed] [Google Scholar]
- Chow C. K., Tappel A. L. An enzymatic protective mechanism against lipid peroxidation damage to lungs of ozone-exposed rats. Lipids. 1972 Aug;7(8):518–524. doi: 10.1007/BF02533017. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J., Einstein A. B., Fefer A. Peroxidase-H2O2-halide system: Cytotoxic effect on mammalian tumor cells. Blood. 1975 Feb;45(2):161–170. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Myeloperoxidase-mediated platelet release reaction. J Clin Invest. 1979 Feb;63(2):177–183. doi: 10.1172/JCI109287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med. 1975 Jun 1;141(6):1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Role of the myeloperoxidase-H2O2-halide system in concanavalin A-induced tumor cell killing by human neutrophils. J Immunol. 1979 Jun;122(6):2605–2610. [PubMed] [Google Scholar]
- Clark R. A., Szot S., Venkatasubramanian K., Schiffmann E. Chemotactic factor inactivation by myeloperoxidase-mediated oxidation of methionine. J Immunol. 1980 Apr;124(4):2020–2026. [PubMed] [Google Scholar]
- Cohen M. S., Ryan J. L., Root R. K. The oxidative metabolism of thioglycollate-elicited mouse peritoneal macrophages: the relationship between oxygen, superoxide and hydrogen peroxide and the effect of monolayer formation. J Immunol. 1981 Sep;127(3):1007–1011. [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Brigham K. L., Kronenberg R. S., Jacob H. S. Complement and leukocyte-mediated pulmonary dysfunction in hemodialysis. N Engl J Med. 1977 Apr 7;296(14):769–774. doi: 10.1056/NEJM197704072961401. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLE L. K., HILL E. G., HOLMAN R. T. The thiobarbituric acid reaction and the autoxidations of polyunsaturated fatty acid methyl esters. Arch Biochem Biophys. 1962 Aug;98:253–261. doi: 10.1016/0003-9861(62)90181-9. [DOI] [PubMed] [Google Scholar]
- DALZIEL K., O'BRIEN J. R. Spectrophotometric studies of the reaction of methaemoglobin with hydrogen peroxide. 1. The formation of methaemoglobin-hydrogen peroxide. Biochem J. 1954 Apr;56(4):648–659. doi: 10.1042/bj0560648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K., O'BRIEN J. R. Spectrophotometric studies of the reaction of methaemoglobin with hydrogen peroxide. 2. The degradation of methaemoglobin by hydrogen peroxide. Biochem J. 1954 Apr;56(4):660–669. doi: 10.1042/bj0560660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R. Oxidative bactericidal mechanisms of polymorphonuclear leukocytes. J Infect Dis. 1975 Mar;131(3):295–303. doi: 10.1093/infdis/131.3.295. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., Johnston R. B., Jr Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood. 1976 Apr;47(4):545–554. [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S. Pyridine nucleotide-dependent generation of hydrogen peroxide by a particulate fraction from human neutrophils. J Immunol. 1981 Mar;126(3):1165–1169. [PubMed] [Google Scholar]
- Denko C. W. Protective role of ceruloplasmin in inflammation. Agents Actions. 1979 Oct;9(4):333–336. doi: 10.1007/BF01970657. [DOI] [PubMed] [Google Scholar]
- Dormandy T. L. Free-radical oxidation and antioxidants. Lancet. 1978 Mar 25;1(8065):647–650. doi: 10.1016/s0140-6736(78)91148-0. [DOI] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Bactericidal activity of metal-mediated peroxide-ascorbate systems. Infect Immun. 1974 Nov;10(5):1077–1083. doi: 10.1128/iai.10.5.1077-1083.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English D., Roloff J. S., Lukens J. N. Regulation of human polymorphonuclear leukocyte superoxide release by cellular responses to chemotactic peptides. J Immunol. 1981 Jan;126(1):165–171. [PubMed] [Google Scholar]
- FRIDOVICH I., HANDLER P. Xanthine oxidase. V. Differential inhibition of the reduction of various electron acceptors. J Biol Chem. 1962 Mar;237:916–921. [PubMed] [Google Scholar]
- Fletcher B. L., Dillard C. J., Tappel A. L. Measurement of fluorescent lipid peroxidation products in biological systems and tissues. Anal Biochem. 1973 Mar;52(1):1–9. doi: 10.1016/0003-2697(73)90327-8. [DOI] [PubMed] [Google Scholar]
- Forman H. J., Fisher A. B. Antioxidant enzymes of rat granular pneumocytes. Constitutive levels and effect of hyperoxia. Lab Invest. 1981 Jul;45(1):1–6. [PubMed] [Google Scholar]
- Forman H. J., York J. L., Fisher A. B. Mechanism for the potentiation of oxygen toxicity by disulfiram. J Pharmacol Exp Ther. 1980 Mar;212(3):452–455. [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- GEORGE P., IRVINE D. H. The reaction between metmyoglobin and hydrogen peroxide. Biochem J. 1952 Nov;52(3):511–517. doi: 10.1042/bj0520511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Hill H. R., Gorman R. R. Unique aspects of the modulation of human neutrophil function by 12-L-hydroperoxy-5,8,10,14-eicosatetraenoic acid. Prostaglandins. 1980 Jan;19(1):71–85. doi: 10.1016/0090-6980(80)90155-0. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J. Mediators of immediate hypersensitivity derived from arachidonic acid. N Engl J Med. 1980 Oct 2;303(14):822–825. doi: 10.1056/NEJM198010023031421. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Edelson H. S., Weissmann G. Ceruloplasmin. A scavenger of superoxide anion radicals. J Biol Chem. 1979 May 25;254(10):4040–4045. [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. R., Stamatoyannopoulos G., Naiman S. C., Kliman M. R., Klebanoff S. J., Austin T., Yoshida A., Robinson G. C. Neutrophil dysfunction, chronic granulomatous disease, and non-spherocytic haemolytic anaemia caused by complete deficiency of glucose-6-phosphate dehydrogenase. Lancet. 1973 Sep 8;2(7828):530–534. doi: 10.1016/s0140-6736(73)92350-7. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980 Apr;23(4):455–463. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Inhibition of collagen gelation by action of the superoxide radical. Arthritis Rheum. 1979 Mar;22(3):251–259. doi: 10.1002/art.1780220307. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Yost F. J., Jr, Fridovich I. Superoxide dismutases of Escherichia coli: intracellular localization and functions. J Bacteriol. 1973 Sep;115(3):987–991. doi: 10.1128/jb.115.3.987-991.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. The protective action of superoxide dismutase on metal-ion catalysed peroxidation of phospholipids. Biochem Biophys Res Commun. 1977 Jul 11;77(1):379–386. doi: 10.1016/s0006-291x(77)80208-8. [DOI] [PubMed] [Google Scholar]
- Hafeman D. G., Lucas Z. J. Polymorphonuclear leukocyte-mediated, antibody-dependent, cellular cytotoxicity against tumor cells: dependence on oxygen and the respiratory burst. J Immunol. 1979 Jul;123(1):55–62. [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981 Jun 15;128(2):347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Heflin A. C., Jr, Brigham K. L. Prevention by granulocyte depletion of increased vascular permeability of sheep lung following endotoxemia. J Clin Invest. 1981 Nov;68(5):1253–1260. doi: 10.1172/JCI110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila R. E., Cabbat F. S., Cohen G. In vivo inhibition of superoxide dismutase in mice by diethyldithiocarbamate. J Biol Chem. 1976 Apr 10;251(7):2182–2185. [PubMed] [Google Scholar]
- Held A. M., Hurst J. K. Ambiguity associated with use of singlet oxygen trapping agents in myeloperoxidase-catalyzed oxidations. Biochem Biophys Res Commun. 1978 Apr 14;81(3):878–885. doi: 10.1016/0006-291x(78)91433-x. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Fox R. B., LeMarbe P. A., Perri R., Repine J. E. Altered oxidative metabolic responses in vitro of alveolar macrophages from asymptomatic cigarette smokers. Am Rev Respir Dis. 1981 Jan;123(1):85–89. doi: 10.1164/arrd.1981.123.1.85. [DOI] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Park B. H., Malawista S. E., Quie P. G., Nelson D. L., Good R. A. Chronic granulomatous disease in females. N Engl J Med. 1970 Jul 30;283(5):217–221. doi: 10.1056/NEJM197007302830501. [DOI] [PubMed] [Google Scholar]
- Janoff A., Sloan B., Weinbaum G., Damiano V., Sandhaus R. A., Elias J., Kimbel P. Experimental emphysema induced with purified human neutrophil elastase: tissue localization of the instilled protease. Am Rev Respir Dis. 1977 Mar;115(3):461–478. doi: 10.1164/arrd.1977.115.3.461. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Varani J., Oliver J., Ward P. A. Immunologic vasculitis in beige mice with deficiency of leukocytic neutral protease. J Immunol. 1979 May;122(5):1807–1811. [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981 Jun;126(6):2365–2369. [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981 Jun;126(6):2365–2369. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Baehner R. L. Improvement of leukocyte bactericidal activity in chronic granulomatous disease. Blood. 1970 Mar;35(3):350–355. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E. Elaboration of toxic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest. 1976 Apr;57(4):836–841. doi: 10.1172/JCI108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEILIN D., HARTREE E. F. Reactions of methaemoglobin and catalase with peroxides and hydrogen donors. Nature. 1954 Apr 17;173(4407):720–723. doi: 10.1038/173720a0. [DOI] [PubMed] [Google Scholar]
- Katzenstein A. L., Bloor C. M., Leibow A. A. Diffuse alveolar damage--the role of oxygen, shock, and related factors. A review. Am J Pathol. 1976 Oct;85(1):209–228. [PMC free article] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem. 1977 Oct 10;252(19):6721–6728. [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Hemolysis and iodination of erythrocyte components by a myeloperoxidase-mediated system. Blood. 1975 May;45(5):699–707. [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Hemolysis and iodination of erythrocyte components by a myeloperoxidase-mediated system. Blood. 1975 May;45(5):699–707. [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Pincus S. H. Hydrogen peroxide utilization in myeloperoxidase-deficient leukocytes: a possible microbicidal control mechanism. J Clin Invest. 1971 Oct;50(10):2226–2229. doi: 10.1172/JCI106718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., White L. R. Iodination defect in the leukocytes of a patient with chronic granulomatous disease of childhood. N Engl J Med. 1969 Feb 27;280(9):460–466. doi: 10.1056/NEJM196902272800902. [DOI] [PubMed] [Google Scholar]
- Koppenol W. H. Reactions involving singlet oxygen and the superoxide anion. Nature. 1976 Jul 29;262(5567):420–421. doi: 10.1038/262420a0. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Species, tissue and subcellular distribution of non Se-dependent glutathione peroxidase activity. J Nutr. 1978 Feb;108(2):211–215. doi: 10.1093/jn/108.2.211. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cohen L. Receptor-mediated regulation of superoxide production in human neutrophils stimulated by phorbol myristate acetate. J Clin Invest. 1981 Nov;68(5):1314–1320. doi: 10.1172/JCI110378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucy J. A. Functional and structural aspects of biological membranes: a suggested structural role for vitamin E in the control of membrane permeability and stability. Ann N Y Acad Sci. 1972 Dec 18;203:4–11. doi: 10.1111/j.1749-6632.1972.tb27849.x. [DOI] [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Effects of superoxide on the erythrocyte membrane. J Biol Chem. 1978 Mar 25;253(6):1838–1845. [PubMed] [Google Scholar]
- Løvstad R. A. The protective action of ceruloplasmin on Fe2+ stimulated lysis of rat erythrocytes. Int J Biochem. 1981;13(2):221–224. doi: 10.1016/0020-711x(81)90159-2. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J Clin Invest. 1975 Mar;55(3):561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Hook E. W. Leukocyte bactericidal activity in chronic granulomatous disease: correlation of bacterial hydrogen peroxide production and susceptibility to intracellular killing. J Bacteriol. 1969 Oct;100(1):531–532. doi: 10.1128/jb.100.1.531-532.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., 2nd, Gadek J. E., Hunninghake G. W., Crystal R. G. Oxidant injury of lung parenchymal cells. J Clin Invest. 1981 Nov;68(5):1277–1288. doi: 10.1172/JCI110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979 May 28;88(2):402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- Matsuda I., Oka Y., Taniguchi N., Furuyama M., Kodama S., Arashima S., Mitsuyama T. Leukocyte glutathione peroxidase deficiency in a male patient with chronic granulomatous disease. J Pediatr. 1976 Apr;88(4 Pt 1):581–583. doi: 10.1016/s0022-3476(76)80010-8. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J. R., Harkin M. M., Johnson K. J., Ward P. A. Suppression by superoxide dismutase of immune-complex--induced pulmonary alveolitis and dermal inflammation. Am J Pathol. 1981 Jan;102(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- McPhail L. C., DeChatelet L. R., Shirley P. S. Further characterization of NADPH oxidase activity of human polymorphonuclear leukocytes. J Clin Invest. 1976 Oct;58(4):774–780. doi: 10.1172/JCI108528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XII. Hydrogen peroxide-myeloperoxidase bactericidal system in the phagocyte. J Bacteriol. 1967 Nov;94(5):1425–1430. doi: 10.1128/jb.94.5.1425-1430.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel C. E. The effects of hyperoxia on red cells as related to tocopherol deficiency. Ann N Y Acad Sci. 1972 Dec 18;203:163–171. doi: 10.1111/j.1749-6632.1972.tb27870.x. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972 May 25;247(10):3170–3175. [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Baxter C. R., Masters B. S. Simultaneous demonstration of phagocytosis-connected oxygen consumption and corresponding NAD(P)H oxidase activity: direct evidence for NADPH as the predominant electron donor to oxygen in phagocytizing human neutrophils. Biochem Biophys Res Commun. 1981 Feb 12;98(3):743–751. doi: 10.1016/0006-291x(81)91175-x. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburger P. E., Chovaniec M. E., Cohen H. J. Activity and activation of the granulocyte superoxide-generating system. Blood. 1980 Jan;55(1):85–92. [PubMed] [Google Scholar]
- Nilsson R., Pick F. M., Bray R. C. EPR studies on reduction of oxygen to superoxide by some biochemical systems. Biochim Biophys Acta. 1969 Oct 7;192(1):145–148. doi: 10.1016/0304-4165(69)90022-1. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Thomas M. J., Lees C. J., McCall C. E. Neutrophil-aggregating activity of monohydroxyeicosatetraenoic acids. Am J Pathol. 1981 Jul;104(1):55–62. [PMC free article] [PubMed] [Google Scholar]
- Oski F. A., Barness L. A. Vitamin E deficiency: a previously unrecognized cause of hemolytic anemia in the premature infant. J Pediatr. 1967 Feb;70(2):211–220. doi: 10.1016/s0022-3476(67)80416-5. [DOI] [PubMed] [Google Scholar]
- Oyanagui Y. Participation of superoxide anions at the prostaglandin phase of carrageenan foot-oedema. Biochem Pharmacol. 1976 Jul 1;25(13):1465–1472. [PubMed] [Google Scholar]
- Parry M. F., Root R. K., Metcalf J. A., Delaney K. K., Kaplow L. S., Richar W. J. Myeloperoxidase deficiency: prevalence and clinical significance. Ann Intern Med. 1981 Sep;95(3):293–301. doi: 10.7326/0003-4819-95-3-293. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Moncalvo S., Rossi F., Romeo D. Enzymatic basis of metabolic stimulation in leucocytes during phagocytosis: the role of activated NADPH oxidase. Arch Biochem Biophys. 1971 Jul;145(1):255–262. doi: 10.1016/0003-9861(71)90034-8. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Tedesco F., Kakinuma K. Studies on the mechanism of metabolic stimulation in polymorphonuclear leucocytes during phagocytosis. II. Presence of the NADPH2 oxidizing activity in a myeloperoxidase-deficient subject. Biochim Biophys Acta. 1975 Apr 7;385(2):387–393. doi: 10.1016/0304-4165(75)90368-2. [DOI] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatt J., O'Brien P. J. Singlet oxygen formation by a peroxidase, H2O2 and halide system. Eur J Biochem. 1979 Jan 15;93(2):323–332. doi: 10.1111/j.1432-1033.1979.tb12826.x. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages--induction by multiple nonphagocytic stimuli. Cell Immunol. 1981 Apr;59(2):301–318. doi: 10.1016/0008-8749(81)90411-1. [DOI] [PubMed] [Google Scholar]
- Puig-Parellada P., Planas J. M. Synovial fluid degradation induced by free radicals. In vitro action of several free radical scavengers and anti-inflammatory drugs. Biochem Pharmacol. 1978 Feb 15;27(4):535–537. doi: 10.1016/0006-2952(78)90390-8. [DOI] [PubMed] [Google Scholar]
- Płonka A., Metodiewa D., Zgirski A., Hilewicz M., Leyko W. ESR evidence of superoxide radical dismutation by human ceruloplasmin. Biochem Biophys Res Commun. 1980 Aug 14;95(3):978–984. doi: 10.1016/0006-291x(80)91569-7. [DOI] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riely C. A., Cohen G., Lieberman M. Ethane evolution: a new index of lipid peroxidation. Science. 1974 Jan 18;183(4121):208–210. doi: 10.1126/science.183.4121.208. [DOI] [PubMed] [Google Scholar]
- Rinaldo J. E., Rogers R. M. Adult respiratory-distress syndrome: changing concepts of lung injury and repair. N Engl J Med. 1982 Apr 15;306(15):900–909. doi: 10.1056/NEJM198204153061504. [DOI] [PubMed] [Google Scholar]
- Rister M., Baehner R. L. The alteration of superoxide dismutase, catalase, glutathione peroxidase, and NAD(P)H cytochrome c reductase in guinea pig polymorphonuclear leukocytes and alveolar macrophages during hyperoxia. J Clin Invest. 1976 Nov;58(5):1174–1184. doi: 10.1172/JCI108570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D., Homan-Müller J. W., Weening R. S. Effect of cytochalasin B on the oxidative metabolism of human peripheral blood granulocytes. Biochem Biophys Res Commun. 1976 Jan 12;68(1):43–50. doi: 10.1016/0006-291x(76)90007-3. [DOI] [PubMed] [Google Scholar]
- Roos D., Weening R. S., Voetman A. A. Protection of human neutrophils against oxidative damage. Agents Actions. 1980 Dec;10(6):528–535. doi: 10.1007/BF02024158. [DOI] [PubMed] [Google Scholar]
- Roos D., Weening R. S., Wyss S. R., Aebi H. E. Protection of human neutrophils by endogenous catalase: studies with cells from catalase-deficient individuals. J Clin Invest. 1980 Jun;65(6):1515–1522. doi: 10.1172/JCI109817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J. A. H2O2 release from human granulocytes during phagocytosis. Relationship to superoxide anion formation and cellular catabolism of H2O2: studies with normal and cytochalasin B-treated cells. J Clin Invest. 1977 Dec;60(6):1266–1279. doi: 10.1172/JCI108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte. J Exp Med. 1979 Jan 1;149(1):27–39. doi: 10.1084/jem.149.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Formation of singlet oxygen by the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1977 Jul 25;252(14):4803–4810. [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Hydroxyl radical generation by polymorphonuclear leukocytes measured by electron spin resonance spectroscopy. J Clin Invest. 1979 Dec;64(6):1725–1729. doi: 10.1172/JCI109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubal W. T., Tappel A. L. Polymerization of proteins induced by free-radical lipid peroxidation. Arch Biochem Biophys. 1966 Jan;113(1):150–155. doi: 10.1016/0003-9861(66)90168-8. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest. 1974 Oct;54(4):1005–1009. doi: 10.1172/JCI107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon S. E., Cline M. J., Schultz J., Lehrer R. I. Myeloperoxidase deficiency. Immunologic study of a genetic leukocyte defect. N Engl J Med. 1970 Jan 29;282(5):250–253. doi: 10.1056/NEJM197001292820505. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Tegner H., Kuhn C., Ohlsson K., Starcher B. C., Pierce J. A. The induction of pulmonary emphysema with human leukocyte elastase. Am Rev Respir Dis. 1977 Sep;116(3):469–475. doi: 10.1164/arrd.1977.116.3.469. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Mehta J., Spilberg I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: effect of metabolic inhibitors and antiinflammatory drugs. Arthritis Rheum. 1979 Jul;22(7):755–763. doi: 10.1002/art.1780220711. [DOI] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Slivka A., LoBuglio A. F., Weiss S. J. A potential role for hypochlorous acid in granulocyte-mediated tumor cell cytotoxicity. Blood. 1980 Feb;55(2):347–350. [PubMed] [Google Scholar]
- Smith D. C., Klebanoff S. J. A uterine fluid-mediated sperm-inhibitory system. Biol Reprod. 1970 Oct;3(2):229–235. doi: 10.1093/biolreprod/3.2.229. [DOI] [PubMed] [Google Scholar]
- Spilberg I., Mehta J., Daughaday C., Simchowitz L. Determination of a specific receptor for formyl-methionyl-leucyl-phenylalanine on th pulmonary alveolar macrophage and its relationship to chemotaxis and superoxide production. J Lab Clin Med. 1981 May;97(5):602–609. [PubMed] [Google Scholar]
- Stendahl O., Lindgren S. Function of granulocytes with deficient myeloperoxidase-mediated iodination in a patient with generalized pustular psoriasis. Scand J Haematol. 1976 Feb;16(2):144–153. doi: 10.1111/j.1600-0609.1976.tb01130.x. [DOI] [PubMed] [Google Scholar]
- Stocks J., Gutteridge J. M., Sharp R. J., Dormandy T. L. The inhibition of lipid autoxidation by human serum and its relation to serum proteins and alpha-tocopherol. Clin Sci Mol Med. 1974 Sep;47(3):223–233. doi: 10.1042/cs0470223. [DOI] [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Jacobs A. A., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XXII. H2O2-dependent decarbosylation and deamination by myeloperoxidase and its relationship to antimicrobial activity. J Reticuloendothel Soc. 1970 Jun;7(6):754–761. [PubMed] [Google Scholar]
- TSEN C. C., COLLIER H. B. The protective action of tocopherol against hemolysis of rat erythrocytes by dialuric acid. Can J Biochem Physiol. 1960 Sep;38:957–964. [PubMed] [Google Scholar]
- Tappel A. L., Dillard C. J. In vivo lipid peroxidation: measurement via exhaled pentane and protection by vitamin E. Fed Proc. 1981 Feb;40(2):174–178. [PubMed] [Google Scholar]
- Tauber A. I., Babior B. M. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977 Aug;60(2):374–379. doi: 10.1172/JCI108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Svvennsen R. J., Franks D. Role of hydrogen peroxide in the cytotoxic reaction of T lymphocytes. Clin Exp Immunol. 1980 Feb;39(2):486–495. [PMC free article] [PubMed] [Google Scholar]
- Thurman R. G., Ley H. G., Scholz R. Hepatic microsomal ethanol oxidation. Hydrogen peroxide formation and the role of catalase. Eur J Biochem. 1972 Feb;25(3):420–430. doi: 10.1111/j.1432-1033.1972.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Vidláková M., Erazímová J., Horký J., Placer Z. Relationship of serum antioxidative activity to tocopherol and serum inhibitor of lipid peroxidation. Clin Chim Acta. 1972 Jan;36(1):61–66. doi: 10.1016/0009-8981(72)90158-1. [DOI] [PubMed] [Google Scholar]
- Waller R. L., Recknagel R. O. Determination of lipid conjugated dienes with tetracyanoethylene-14C: significance for study of the pathology of lipid peroxidation. Lipids. 1977 Nov;12(11):914–921. doi: 10.1007/BF02533311. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. An oxygen-dependent mechanism of neutrophil-mediated cytotoxicity. Blood. 1980 Jun;55(6):1020–1024. [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F., Kessler H. B. Oxidative mechanisms of monocyte-mediated cytotoxicity. Proc Natl Acad Sci U S A. 1980 Jan;77(1):584–587. doi: 10.1073/pnas.77.1.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Rustagi P. K., LoBuglio A. F. Human granulocyte generation of hydroxyl radical. J Exp Med. 1978 Feb 1;147(2):316–323. doi: 10.1084/jem.147.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. The role of superoxide in the destruction of erythrocyte targets by human neutrophils. J Biol Chem. 1980 Oct 25;255(20):9912–9917. [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatti M., Rossi F., Patriarca P. The H2O2-production by polymorphonuclear leukocytes during phagocytosis. Experientia. 1968 Jul 15;24(7):669–670. doi: 10.1007/BF02138302. [DOI] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Ostrowiski W., Naskalski J., Sznajd J. Myeloperoxidase of human leukaemic leucocytes. Oxidation of amino acids in the presence of hydrogen peroxide. Eur J Biochem. 1968 May;4(4):540–547. doi: 10.1111/j.1432-1033.1968.tb00246.x. [DOI] [PubMed] [Google Scholar]