Abstract
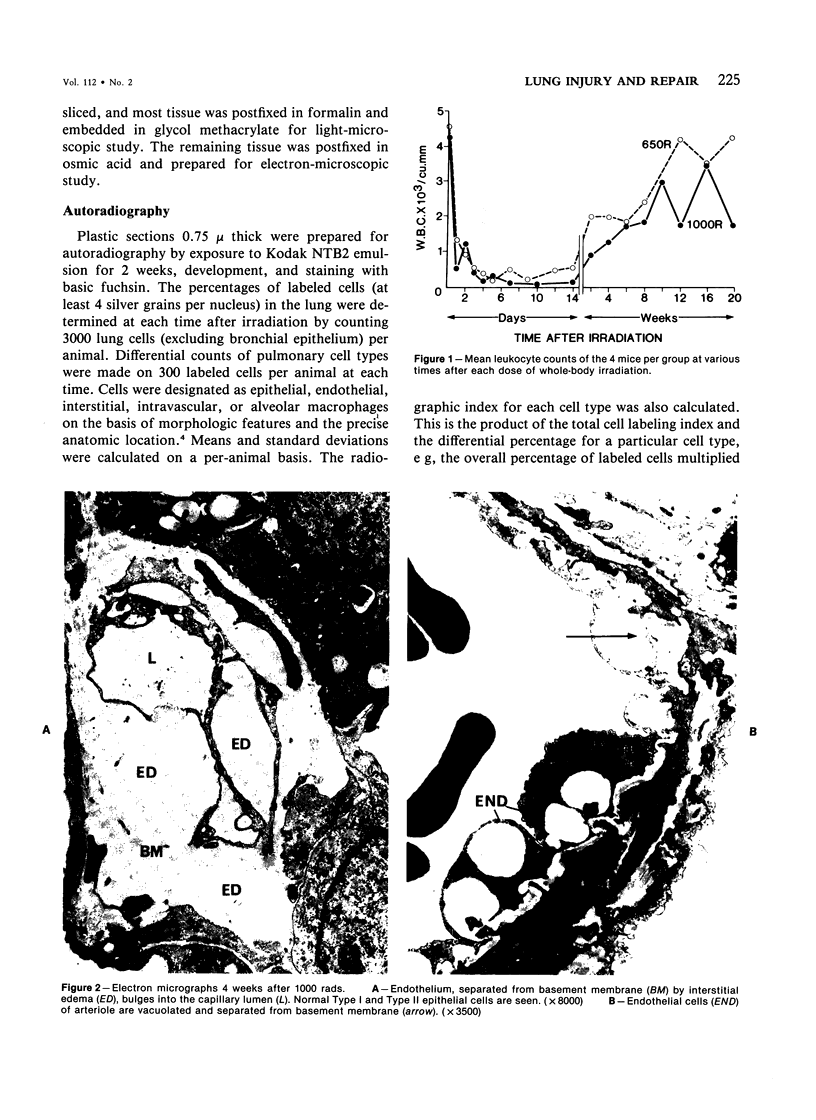
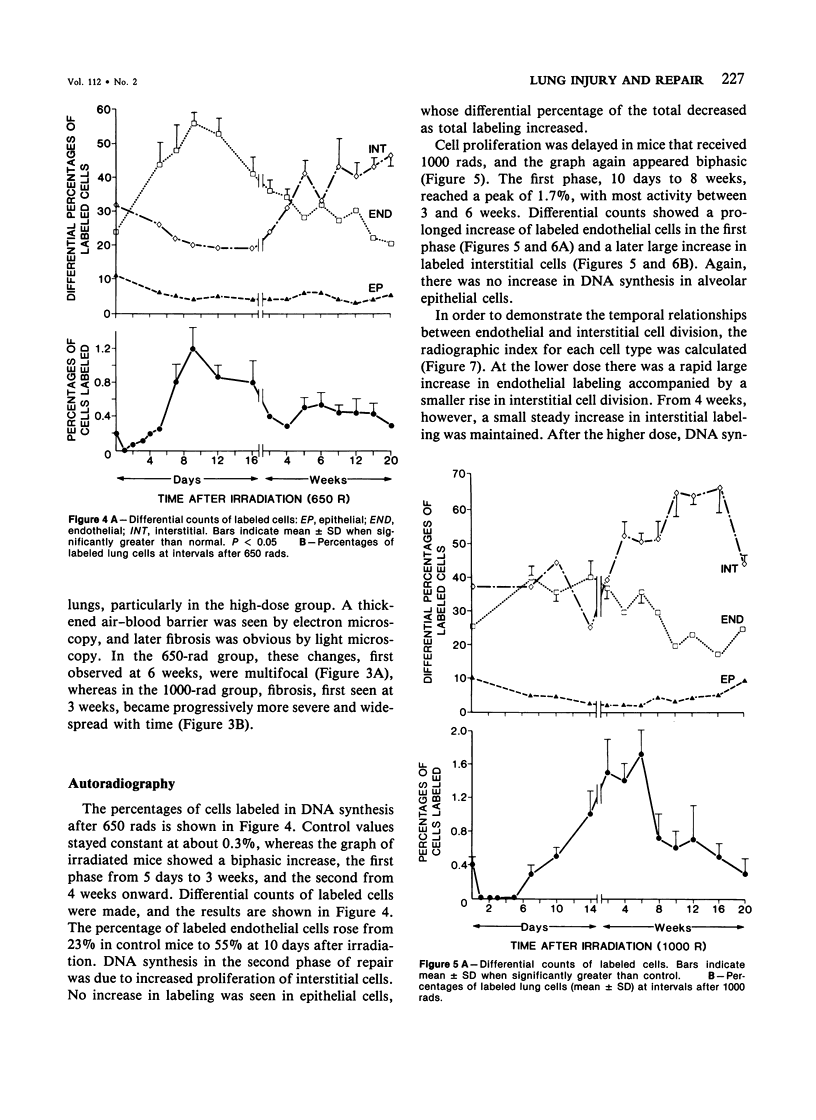
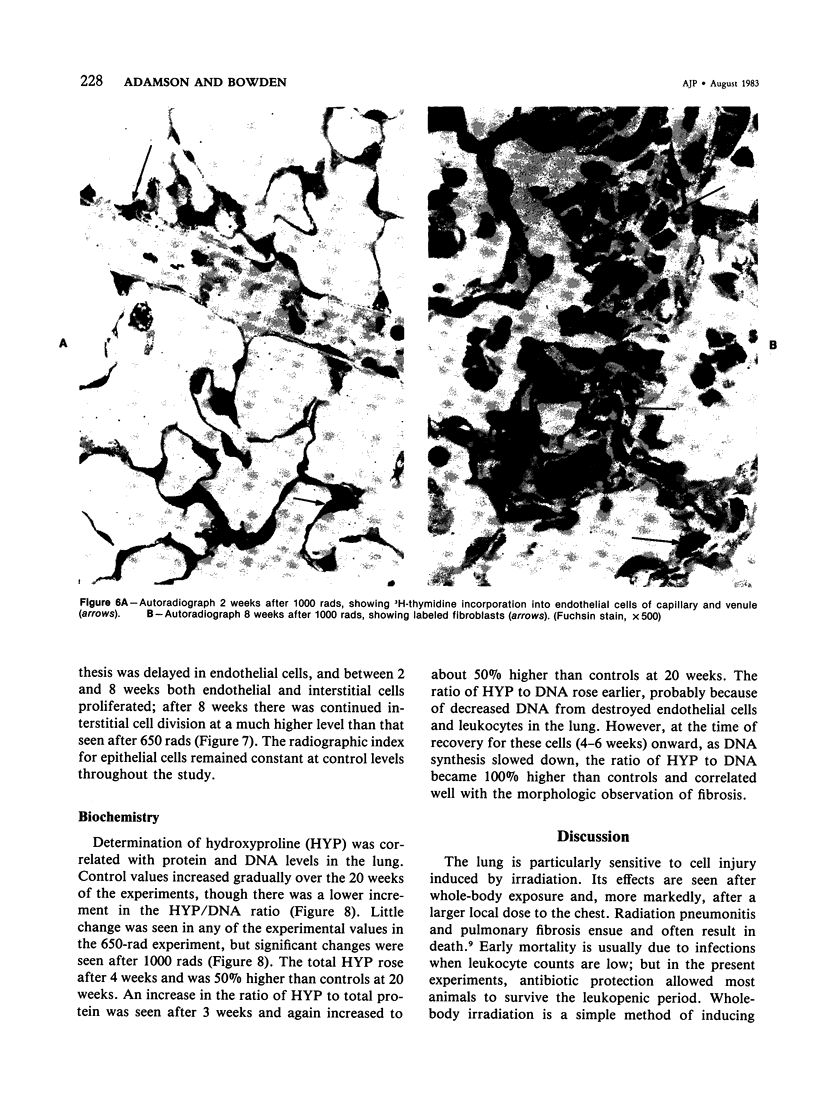
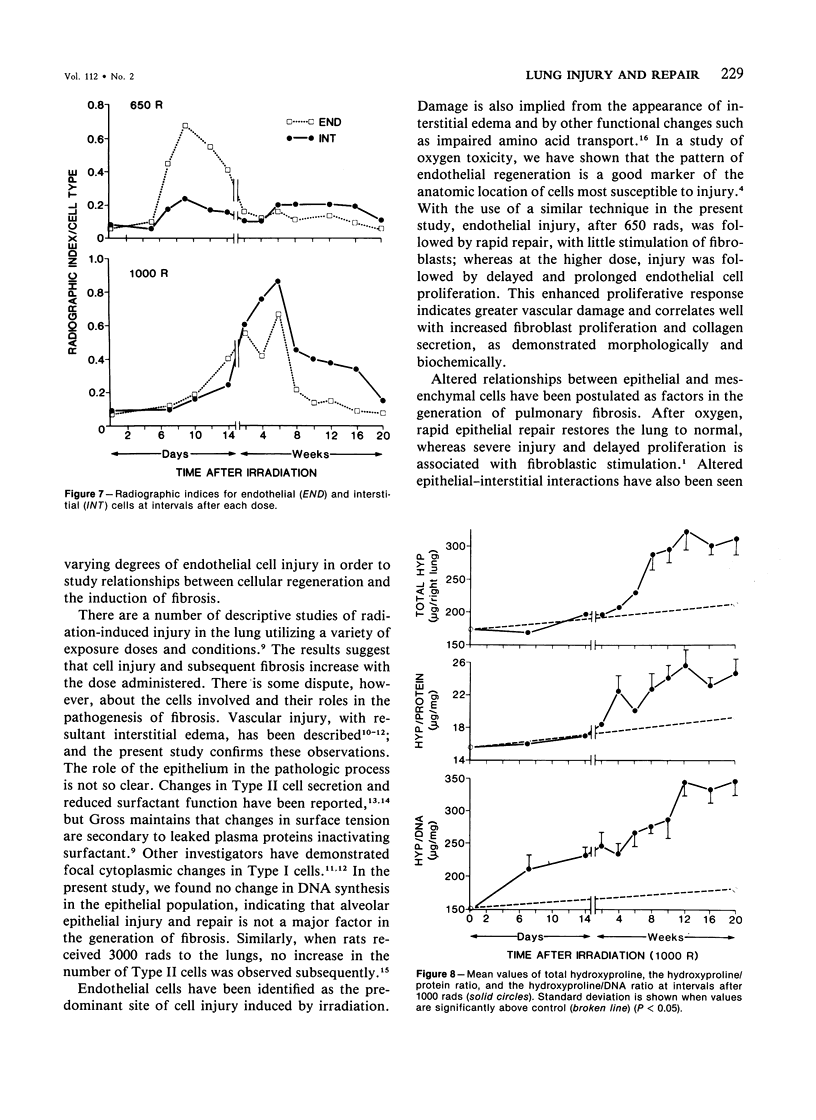
Cytokinetic relationships between endothelial cells and fibroblasts during lung injury and repair in mice have been studied in a morphologic, autoradiographic, and biochemical study following whole body irradiation. After 650 rads, endothelial injury accompanied by interstitial edema was seen between weeks 1 and 2. The cell labeling curve had two components: predominant endothelial labeling to 3 weeks, then a smaller rise in DNA synthesis in interstitial cells. There was focal fibrosis but little change in total hydroxyproline to 20 weeks. After 1000 rads, cell injury, still confined to the endothelium, was more severe and lasted up to 6 weeks. Increased DNA synthesis occurred in the endothelium between Weeks 2 and 8 and in interstitial cells from Week 3 to 16, when total hydroxyproline was significantly elevated and many fibrotic areas were seen in the lung. The results indicate that acute endothelial injury may be rapidly repaired with little fibroblastic stimulation, whereas severe or prolonged injury with delayed regeneration disturbs endothelial-mesenchymal relationships. This may be a key factor in promoting fibroblast proliferation and the deposition of collagen.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Bleomycin-induced injury and metaplasia of alveolar type 2 cells. Relationship of cellular responses to drug presence in the lung. Am J Pathol. 1979 Aug;96(2):531–544. [PMC free article] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H., Wyatt J. P. A pathway to pulmonary fibrosis: an ultrastructural study of mouse and rat following radiation to the whole body and hemithorax. Am J Pathol. 1970 Mar;58(3):481–498. [PMC free article] [PubMed] [Google Scholar]
- Adamson Y. I., Bowden D. H. Pulmonary injury and repair. Organ culture studies of murine lung after oxygen. Arch Pathol Lab Med. 1976 Dec;100(12):640–643. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Endothelial regeneration as a marker of the differential vascular responses in oxygen-induced pulmonary edema. Lab Invest. 1974 Mar;30(3):350–357. [PubMed] [Google Scholar]
- Brody A. R., Soler P., Basset F., Haschek W. M., Witschi H. Epithelial-mesenchymal associations of cells in human pulmonary fibrosis and in BHT-oxygen-induced fibrosis in mice. Exp Lung Res. 1981 Aug;2(3):207–220. doi: 10.3109/01902148109052316. [DOI] [PubMed] [Google Scholar]
- Coultas P. G., Ahier R. G., Field S. B. Effects of neutron and X irradiation on cell proliferation in mouse lung. Radiat Res. 1981 Mar;85(3):516–528. [PubMed] [Google Scholar]
- Gajdusek C. M., Schwartz S. M. Ability of endothelial cells to condition culture medium. J Cell Physiol. 1982 Jan;110(1):35–42. doi: 10.1002/jcp.1041100107. [DOI] [PubMed] [Google Scholar]
- Gross N. J. The pathogenesis of radiation-induced lung damage. Lung. 1981;159(3):115–125. doi: 10.1007/BF02713907. [DOI] [PubMed] [Google Scholar]
- Haudenschild C. C., Schwartz S. M. Endothelial regeneration. II. Restitution of endothelial continuity. Lab Invest. 1979 Nov;41(5):407–418. [PubMed] [Google Scholar]
- Kwock L., Douglas W. H., Lin P. S., Baur W. E., Fanburg B. L. Endothelial cell damage after gamma-irradiation in vitro: impaired uptake of alpha-aminoisobutyric acid. Am Rev Respir Dis. 1982 Jan;125(1):95–99. doi: 10.1164/arrd.1982.125.1.95. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Madri J. A., Stenn K. S. Aortic endothelial cell migration. I. Matrix requirements and composition. Am J Pathol. 1982 Feb;106(2):180–186. [PMC free article] [PubMed] [Google Scholar]
- Maisin J. R. The ultrastructure of the lung of mice exposed to a supra-lethal dose of ionizing radiation on the thorax. Radiat Res. 1970 Nov;44(2):545–564. [PubMed] [Google Scholar]
- Penney D. P., Shapiro D. L., Rubin P., Finkelstein J., Siemann D. W. Effects of radiation on the mouse lung and potential induction of radiation pneumonitis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;37(3):327–336. doi: 10.1007/BF02892581. [DOI] [PubMed] [Google Scholar]
- Phillips T. L. An ultrastructural study of the development of radiation injury in the lung. Radiology. 1966 Jul;87(1):49–54. doi: 10.1148/87.1.49. [DOI] [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial regeneration. III. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981 Apr;44(4):301–308. [PubMed] [Google Scholar]
- Willems C., Astaldi G. C., De Groot P. G., Janssen M. C., Gonsalvez M. D., Zeijlemaker W. P., Van Mourik J. A., Van Aken W. G. Media conditioned by cultured human vascular endothelial cells inhibit the growth of vascular smooth muscle cells. Exp Cell Res. 1982 May;139(1):191–197. doi: 10.1016/0014-4827(82)90332-9. [DOI] [PubMed] [Google Scholar]






