Abstract
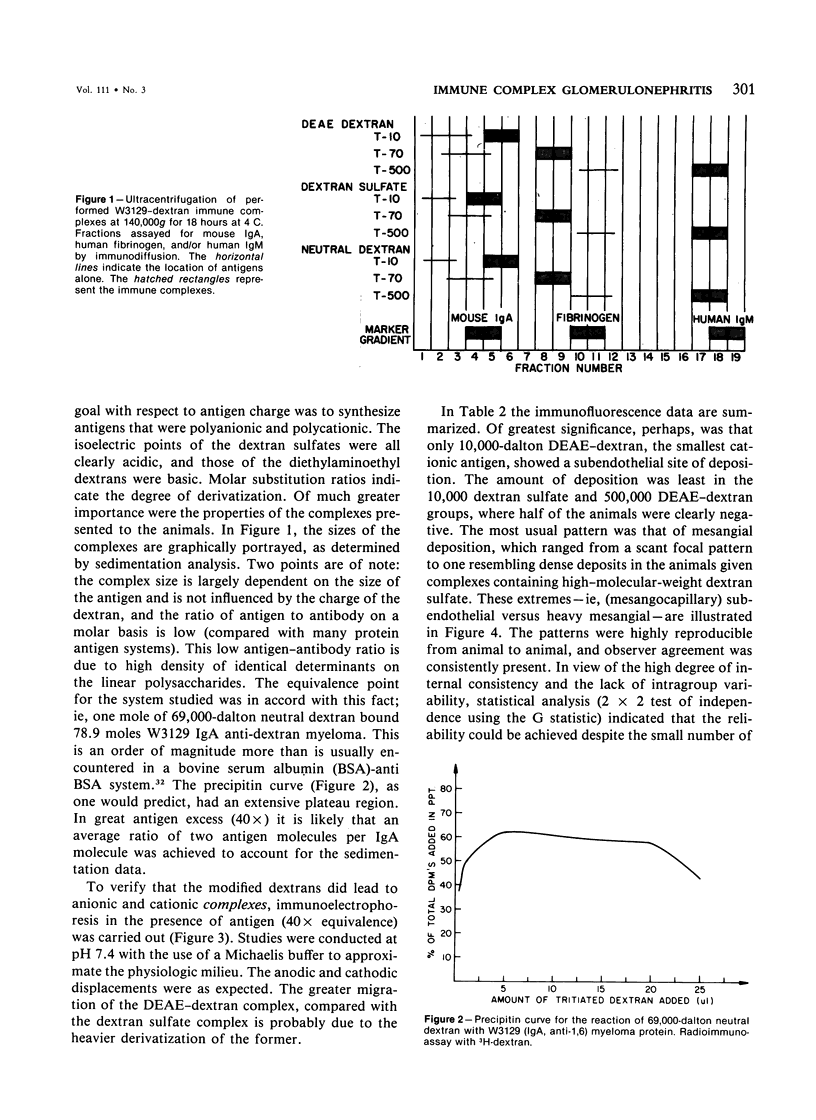
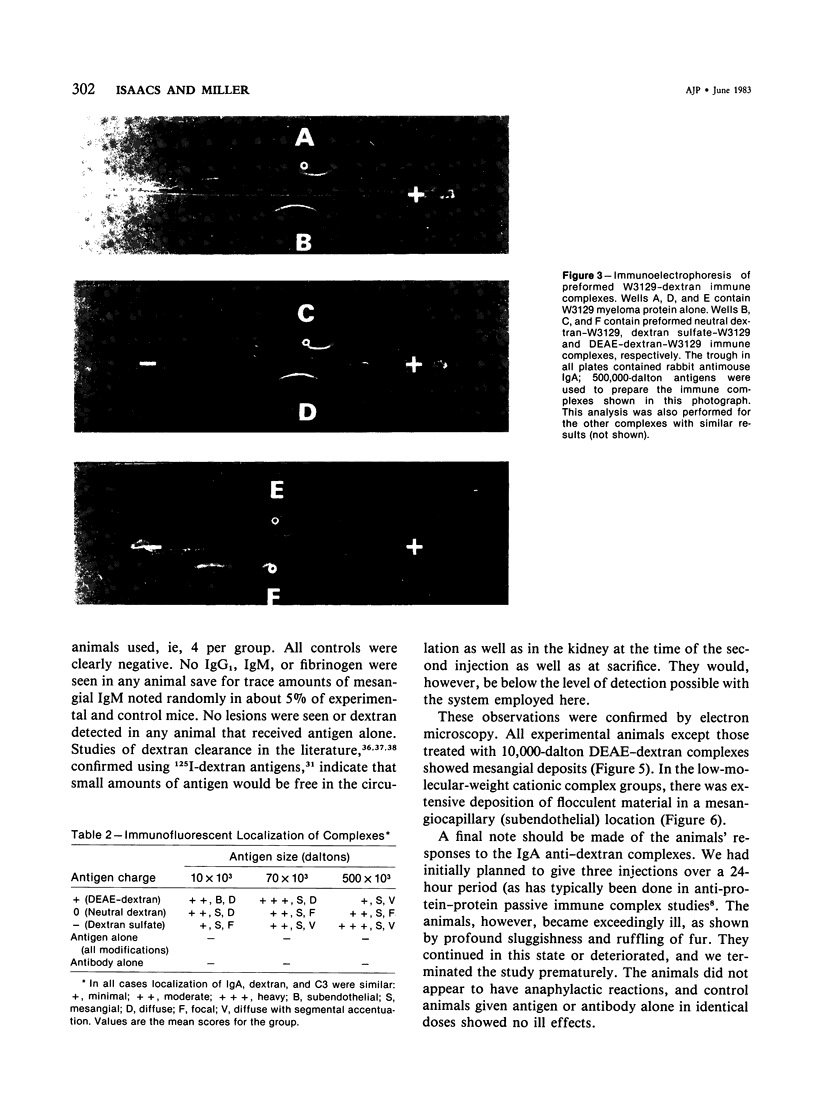
Utilizing dextrans of restricted sizes (10,000, 70,000, 500,000 daltons), modified with regard to charge (neutral, polycationic, polyanionic) and an anti-dextran murine IgA myeloma, W3129, the authors have examined a model that may be used in the study of the combined effect of size and charge on renal deposition of immune complexes. Polycationic DEAE dextran complexes, using the 10,000 dalton antigen, showed a mesangiocapillary pattern of deposition. The other antigens showed focal to diffuse mesangial localization of varying degree. This indicates the potential usefulness of this system in examining the factors important in glomerular immune injury. The relevance to other observations, importance of polysaccharide antigens, and role in circulating versus in situ or "planted" immune complex models are considered.
Full text
PDF


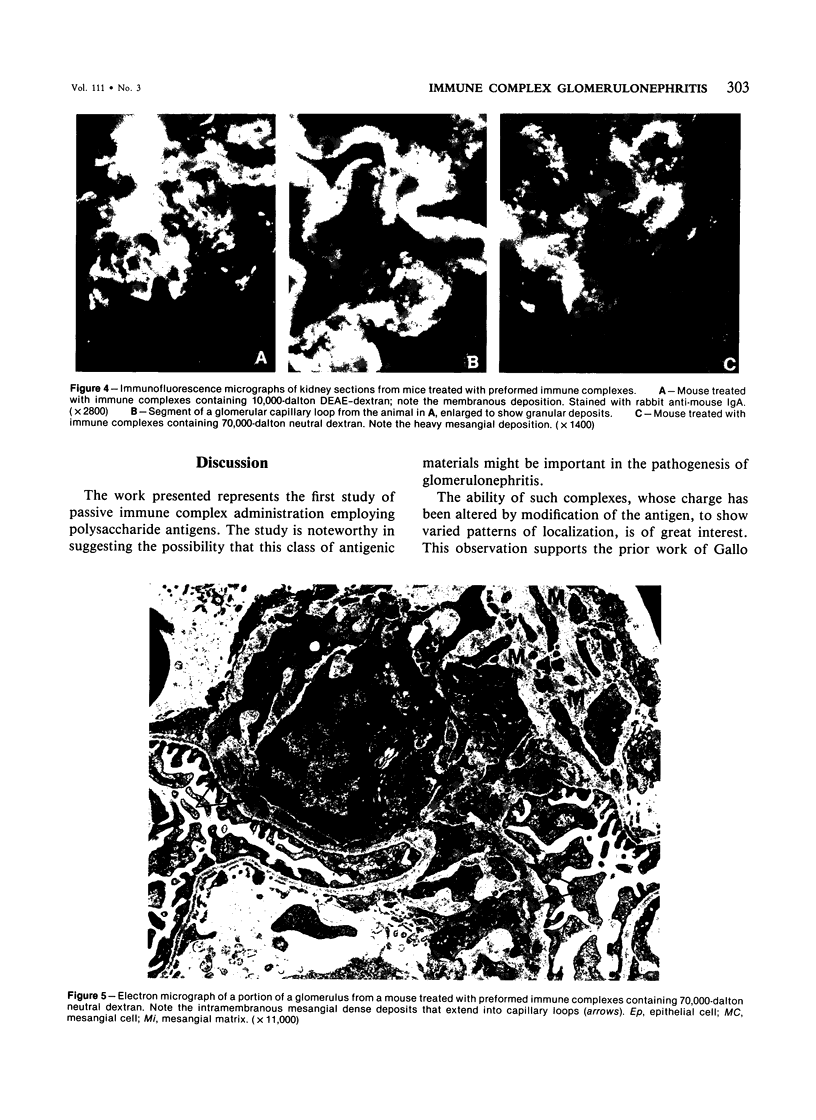



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batsford S. R., Takamiya M., Vogt A. A model of in situ immune complex glomerulonephritis in the rat employing cationized ferritin. Clin Nephrol. 1980 Nov;14(5):211–216. [PubMed] [Google Scholar]
- Bennett C. M., Glassock R. J., Chang R. L., Deen W. M., Robertson C. R., Brenner B. M., Troy J. L., ueki I. R., Rasmussen B. Permselectivity of the glomerular capillary wall. Studies of experimental glomerulonephritis in the rat using dextran sulfate. J Clin Invest. 1976 May;57(5):1287–1294. doi: 10.1172/JCI108396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrer M. P., Baylis C., Humes H. D., Glassock R. J., Robertson C. R., Brenner B. M. Permselectivity of the glomerular capillary wall. Facilitated filtration of circulating polycations. J Clin Invest. 1978 Jan;61(1):72–78. doi: 10.1172/JCI108927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border W. A., Kamil E. S., Ward H. J., Cohen A. H. Antigenic changes as a determinant of immune complex localization in the rat glomerulus. Lab Invest. 1981 Nov;45(5):442–449. [PubMed] [Google Scholar]
- Carson D., Weigert M. Immunochemical analysis of the cross-reacting idiotypes of mouse myeloma proteins with anti-dextran activity and normal anti-dextran antibody. Proc Natl Acad Sci U S A. 1973 Jan;70(1):235–239. doi: 10.1073/pnas.70.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. Distribution of annionic sites in glomerular basement membranes: their possible role in filtration and attachment. Proc Natl Acad Sci U S A. 1976 May;73(5):1646–1650. doi: 10.1073/pnas.73.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceska M. The synthesis of cross-linked dextran and its enzymatic hydrolysis. Experientia. 1971;27(11):1263–1263. doi: 10.1007/BF02136675. [DOI] [PubMed] [Google Scholar]
- Chang R. L., Deen W. M., Robertson C. R., Bennett C. M., Glassock R. J., Brenner B. M., Troy J. L., Ueki I. F., Rasmussen B. Permselectivity of of the glomerular capillary wall. Studies of experimental glomerulonephritis in the rat using neutral dextran. J Clin Invest. 1976 May;57(5):1272–1286. doi: 10.1172/JCI108395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. L., Ueki I. F., Troy J. L., Deen W. M., Robertson C. R., Brenner B. M. Permselectivity of the glomerular capillary wall to macromolecules. II. Experimental studies in rats using neutral dextran. Biophys J. 1975 Sep;15(9):887–906. doi: 10.1016/S0006-3495(75)85863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comper W. D., Laurent T. C. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978 Jan;58(1):255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- Couser W. G., Salant D. J. In situ immune complex formation and glomerular injury. Kidney Int. 1980 Jan;17(1):1–13. doi: 10.1038/ki.1980.1. [DOI] [PubMed] [Google Scholar]
- Couser W. G., Steinmuller D. R., Stilmant M. M., Salant D. J., Lowenstein L. M. Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest. 1978 Dec;62(6):1275–1287. doi: 10.1172/JCI109248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON F. J., FELDMAN J. D., VAZQUEZ J. J. Experimental glomerulonephritis. The pathogenesis of a laboratory model resembling the spectrum of human glomerulonephritis. J Exp Med. 1961 May 1;113:899–920. doi: 10.1084/jem.113.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. R., Caulin-Glaser T., Lamm M. E. Charge of circulating immune complexes as a factor in glomerular basement membrane localization in mice. J Clin Invest. 1981 May;67(5):1305–1313. doi: 10.1172/JCI110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germuth F. G., Jr, Rodriguez E., Lorelle C. A., Trump E. I., Milano L. L., Wise O. Passive immune complex glomerulonephritis in mice: models for various lesions found in human disease. II. Low avidity complexes and diffuse proliferative glomerulonephritis with subepithelial deposits. Lab Invest. 1979 Oct;41(4):366–371. [PubMed] [Google Scholar]
- Germuth F. G., Jr, Rodriguez E., Lorelle C. A., Trump E. I., Milano L., Wise O. Passive immune complex glomerulonephritis in mice: models for various lesions found in human disease. I. High avidity complexes and mesangiopathic glomerulonephritis. Lab Invest. 1979 Oct;41(4):360–365. [PubMed] [Google Scholar]
- Germuth F. G., Jr, Senterfit L. B., Dreesman G. R. Immune complex disease. V. The nature of the circulating complexes associated with glomerular alterations in the chronic BSA-rabbit system. Johns Hopkins Med J. 1972 Jun;130(6):344–357. [PubMed] [Google Scholar]
- Golbus S. M., Wilson C. B. Experimental glomerulonephritis induced by in situ formation of immune complexes in glomerular capillary wall. Kidney Int. 1979 Aug;16(2):148–157. doi: 10.1038/ki.1979.116. [DOI] [PubMed] [Google Scholar]
- Haakenstad A. O., Striker G. E., Mannik M. The glomerular deposition of soluble immune complexes prepared with reduced and alkylated antibodies and with intact antibodies in mice. Lab Invest. 1976 Sep;35(3):293–301. [PubMed] [Google Scholar]
- Isaacs K. L., Miller F. Role of antigen size and charge in immune complex glomerulonephritis. Lab Invest. 1982 Aug;47(2):198–205. [PubMed] [Google Scholar]
- Isaacs K., Miller F., Lane B. Experimental model for IgA nephropathy. Clin Immunol Immunopathol. 1981 Sep;20(3):419–426. doi: 10.1016/0090-1229(81)90152-5. [DOI] [PubMed] [Google Scholar]
- Keck K. An easy method for labelling polysaccharides with radioactive iodine. Immunochemistry. 1972 Mar;9(3):359–360. doi: 10.1016/0019-2791(72)90098-5. [DOI] [PubMed] [Google Scholar]
- Koyama A., Niwa Y., Shigematsu H., Taniguchi M., Tada T. Studies on passive serum sickness. II. Factors determining the localization of antigen-antibody complexes in the murine renal glomerulus. Lab Invest. 1978 Mar;38(3):253–262. [PubMed] [Google Scholar]
- MCCLUSKEY R. T., BENACERRAF B., POTTER J. L., MILLER F. The pathologic effects of intravenously administered soluble antigen-antibody complexes. I. Passive serum sickness in mice. J Exp Med. 1960 Feb 1;111:181–194. doi: 10.1084/jem.111.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOKRASCH L. C. Analysis of hexose phosphates and sugar mixtures with the anthrone reagent. J Biol Chem. 1954 May;208(1):55–59. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Matsuuchi L., Morrison S. L. Estimation of antibodies specific for dextran. J Immunol. 1978 Sep;121(3):962–965. [PubMed] [Google Scholar]
- McKernan W. M., Ricketts C. R. A basic derivative of dextran and its interaction with serum albumin. Biochem J. 1960 Jul;76(1):117–120. doi: 10.1042/bj0760117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A. F., Blau E., Vernier R. L. Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab Invest. 1970 Dec;23(6):649–657. [PubMed] [Google Scholar]
- Miller F. The carbohydrate moieties of mouse immunoglobulins: composition and evidence against a role in transplacental transport. J Immunol. 1971 Oct;107(4):1161–1167. [PubMed] [Google Scholar]
- Purtell J. N., Pesce A. J., Clyne D. H., Miller W. C., Pollak V. E. Isoelectric point of albumin: effect on renal handling of albumin. Kidney Int. 1979 Sep;16(3):366–376. doi: 10.1038/ki.1979.139. [DOI] [PubMed] [Google Scholar]
- Rennke H. G., Cotran R. S., Venkatachalam M. A. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J Cell Biol. 1975 Dec;67(3):638–646. doi: 10.1083/jcb.67.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C. F., Jr, Rennke H. G., Humes H. D. Acute renal failure induced by diethylaminoethyl dextran: importance of cationic charge. Kidney Int. 1981 Mar;19(3):424–430. doi: 10.1038/ki.1981.35. [DOI] [PubMed] [Google Scholar]
- Sugii S., Takeo K., Kabat E. A. Binding constants of NZB myeloma antidextrans for dextrans and isomaltose oligosaccharides determined by affinity electrophoresis. J Immunol. 1979 Sep;123(3):1162–1168. [PubMed] [Google Scholar]
- Takeo K., Kabat E. A. Binding constants of dextrans and isomaltose oligosaccharides to dextran-specific myeloma proteins determined by affinity electrophoresis. J Immunol. 1978 Dec;121(6):2305–2310. [PubMed] [Google Scholar]
- Van Damme B. J., Fleuren G. J., Bakker W. W., Vernier R. L., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab Invest. 1978 Apr;38(4):502–510. [PubMed] [Google Scholar]
- van Es L. A., Blok A. P., Schoenfield L., Glassock R. J. Chronic nephritis induced by antibodies reacting with glomerular-bound immune complexes. Kidney Int. 1977 Feb;11(2):106–115. doi: 10.1038/ki.1977.15. [DOI] [PubMed] [Google Scholar]