Abstract
A knowledge of the distribution of oxygen tension (PO2) and vascularization in neoplasia has been fundamental to understanding relationships between tumor growth, hypoxia, and therapy. We have combined recessed oxygen microcathode and freeze-substitution techniques to correlate in situ PO2 profiles and morphologic features in 7,12-dimethylbenz(a)anthracene (DMBA) tumors in rats. Overlying connective tissue of transplanted tumor was exposed by a 1-2 mm incision and a cross-stitch pattern demarcated electrode puncture sites for histologic reference. Three buffered salt solutions (BSS) with different PO2 were each allowed to flow through a well over the tumor where electrodes were placed for calibration. Zero electrode oxygen current was recorded from a buffered yeast-agar mixture of zero torr. PO2 was recorded at 5-mu intervals to approximately 1-2 mm. Atmospheric contamination was eliminated by continuous well flow of BSS, 30 torr. Finally, the tumor and surrounding tissues were quick-frozen in vivo with Freon 22 and liquid nitrogen. The tissue block was freeze-substituted and sectioned. PO2 profiles were superimposed onto correspondingly scaled photomicrographs. A viable periphery with a PO2 range of 50-82 torr and a transition to necrotic areas of PO2, 2-13 torr were observed. This transition was characterized by PO2 gradients within distances of 50-300 mu at variable puncture depths. This technique should be useful in further studies of growth, necrosis, and therapy.
Full text
PDF
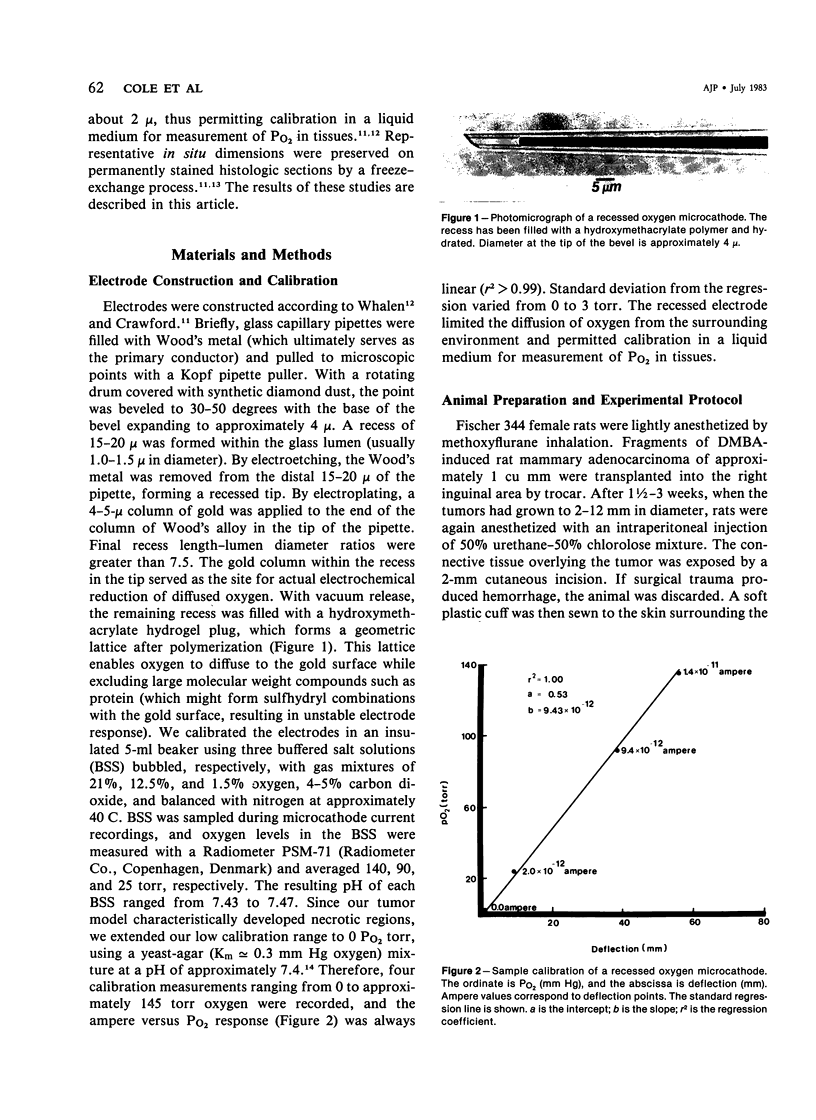
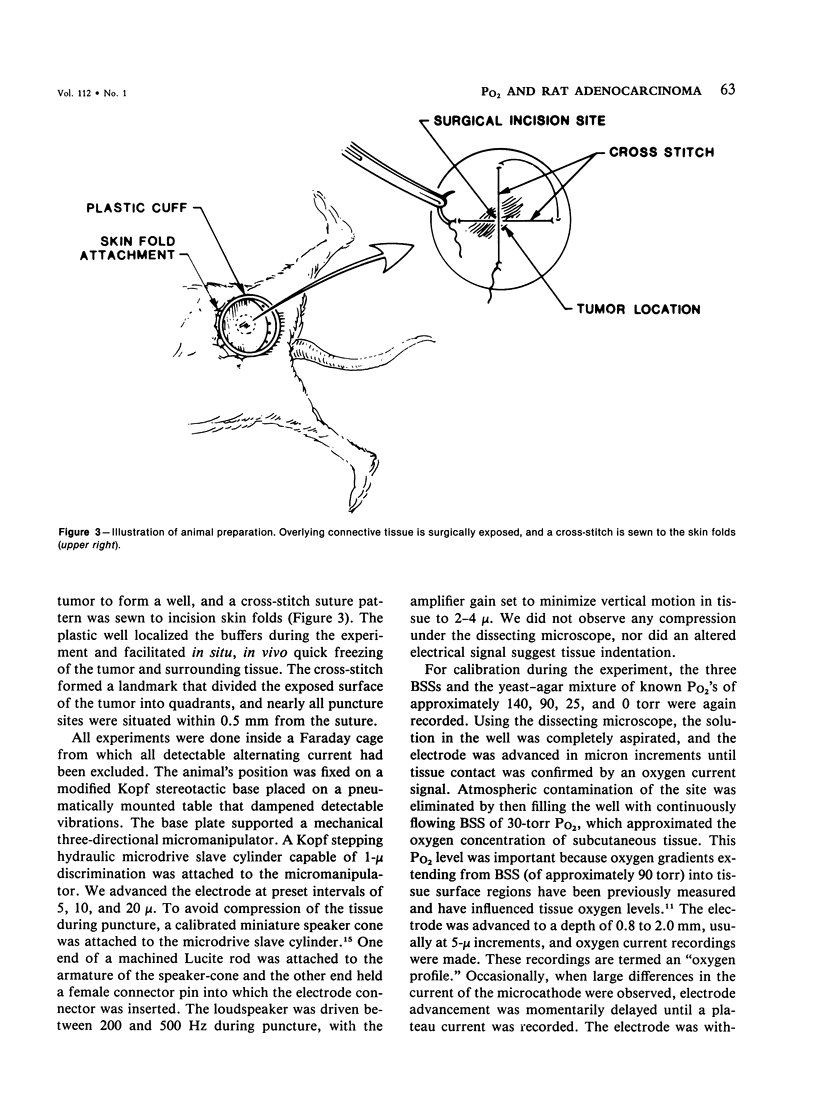
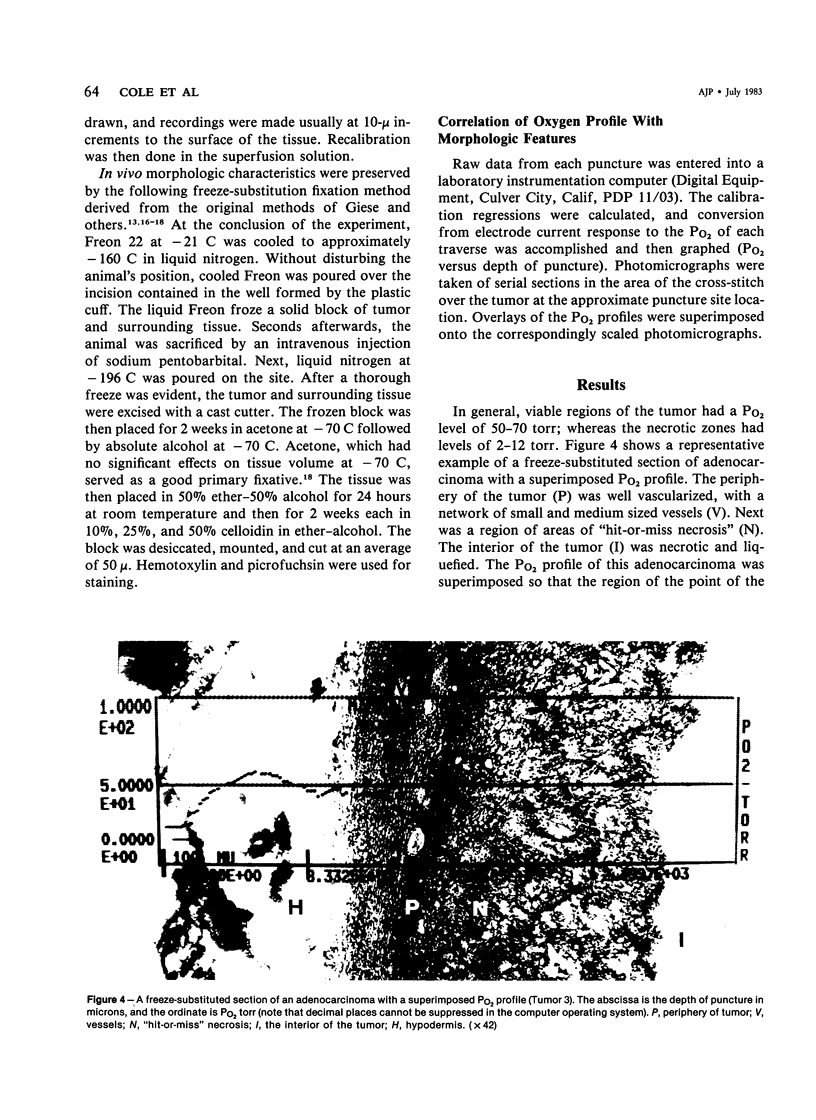
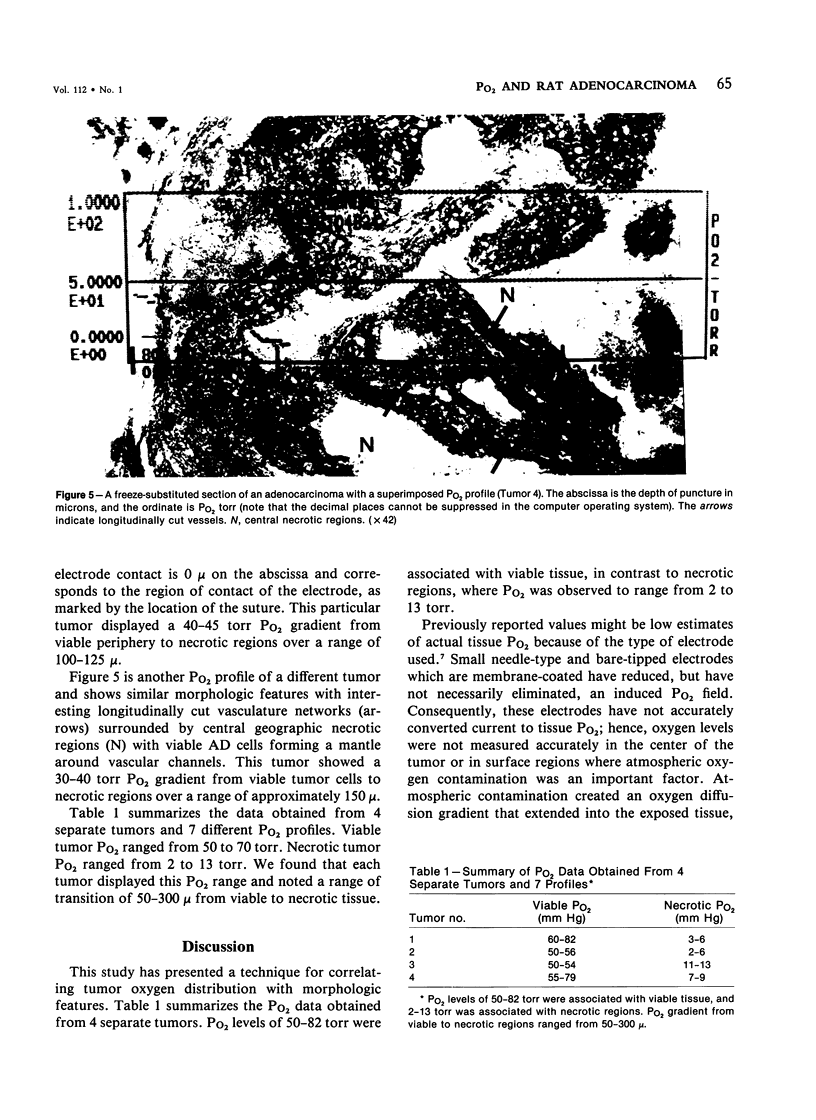


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CATER D. B., PHILLIPS A. F., SILVER I. A. Apparatus and techniques for the measurement of oxidation-reduction potentials, pH and oxygen tension in vivo. Proc R Soc Lond B Biol Sci. 1956 Mar 26;146(923):289–297. doi: 10.1098/rspb.1957.0012. [DOI] [PubMed] [Google Scholar]
- CATER D. B., SILVER I. A. Quantitative measurements of oxygen tension in normal tissues and in the tumours of patients before and after radiotherapy. Acta radiol. 1960 Mar;53:233–256. doi: 10.3109/00016926009171671. [DOI] [PubMed] [Google Scholar]
- Crawford D. W., Back L. H., Cole M. A. In vivo oxygen transport in the normal rabbit femoral arterial wall. J Clin Invest. 1980 Jun;65(6):1498–1508. doi: 10.1172/JCI109815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Monte U. Changes in oxygen tension in Yoshida ascites hepatoma during growth. Proc Soc Exp Biol Med. 1967 Jul;125(3):853–856. doi: 10.3181/00379727-125-32222. [DOI] [PubMed] [Google Scholar]
- GIESE J. ACUTE HYPERTENSIVE VASCULAR DISEASE. 2. STUDIES ON VASCULAR REACTION PATTERNS AND PERMEABILITY CHANGES BY MEANS OF VITAL MICROSCOPY AND COLLOIDAL TRACER TECHNIQUE. Acta Pathol Microbiol Scand. 1964;62:497–515. doi: 10.1111/apm.1964.62.4.497. [DOI] [PubMed] [Google Scholar]
- GRAY L. H., CONGER A. D., EBERT M., HORNSEY S., SCOTT O. C. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953 Dec;26(312):638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- Gullino P. M., Grantham F. H., Courtney A. H. Utilization of oxygen by transplanted tumors in vivo. Cancer Res. 1967 Jun;27(6):1020–1030. [PubMed] [Google Scholar]
- Günther H., Vaupel P., Metzger H., Thews G. Stationäre Vereilung der O 2 -Drucke im Tumorgewebe (DS-Carcinosarkom). I. Messungen in vivo unter Verwendung von Gold-Mikroelektroden. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1972;77(1):26–39. doi: 10.1007/BF00284351. [DOI] [PubMed] [Google Scholar]
- Kanabus E. W., Feldstein C., Crawford D. W. Excursion of vibrating microelectrodes in tissue. J Appl Physiol Respir Environ Exerc Physiol. 1980 Apr;48(4):737–741. doi: 10.1152/jappl.1980.48.4.737. [DOI] [PubMed] [Google Scholar]
- Nair P., Spande J. I., Whalen W. J. Marking the tip location of PO2 microelectrodes or glass micropipettes. J Appl Physiol Respir Environ Exerc Physiol. 1980 Nov;49(5):916–918. doi: 10.1152/jappl.1980.49.5.916. [DOI] [PubMed] [Google Scholar]
- STAUB N. C., STOREY W. F. Relation between morphological and physiological events in lung studied by rapid freezing. J Appl Physiol. 1962 May;17:381–390. doi: 10.1152/jappl.1962.17.3.381. [DOI] [PubMed] [Google Scholar]
- Schneiderman G., Goldstick T. K. Oxygen electrode design criteria and performance characteristics: recessed cathode. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):145–154. doi: 10.1152/jappl.1978.45.1.145. [DOI] [PubMed] [Google Scholar]
- Vaupel P. Hypoxia in neoplastic tissue. Microvasc Res. 1977 May;13(3):399–408. doi: 10.1016/0026-2862(77)90106-6. [DOI] [PubMed] [Google Scholar]
- West J. R., Deadwyler S. A., Cotman C. W., Lynch G. A dual marking technique for microelectrode tracks and localization recording sites. Electroencephalogr Clin Neurophysiol. 1975 Oct;39(4):407–410. doi: 10.1016/0013-4694(75)90104-2. [DOI] [PubMed] [Google Scholar]
- Whalen W. J., Nair P., Ganfield R. A. Measurements of oxygen tension in tissues with a micro oxygen electrode. Microvasc Res. 1973 May;5(3):254–262. doi: 10.1016/0026-2862(73)90035-6. [DOI] [PubMed] [Google Scholar]
- Whalen W. J., Riley J., Nair P. A microelectrode for measuring intracellular PO2. J Appl Physiol. 1967 Nov;23(5):798–801. doi: 10.1152/jappl.1967.23.5.798. [DOI] [PubMed] [Google Scholar]





