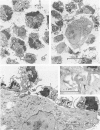
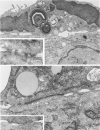
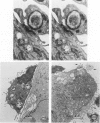
Abstract
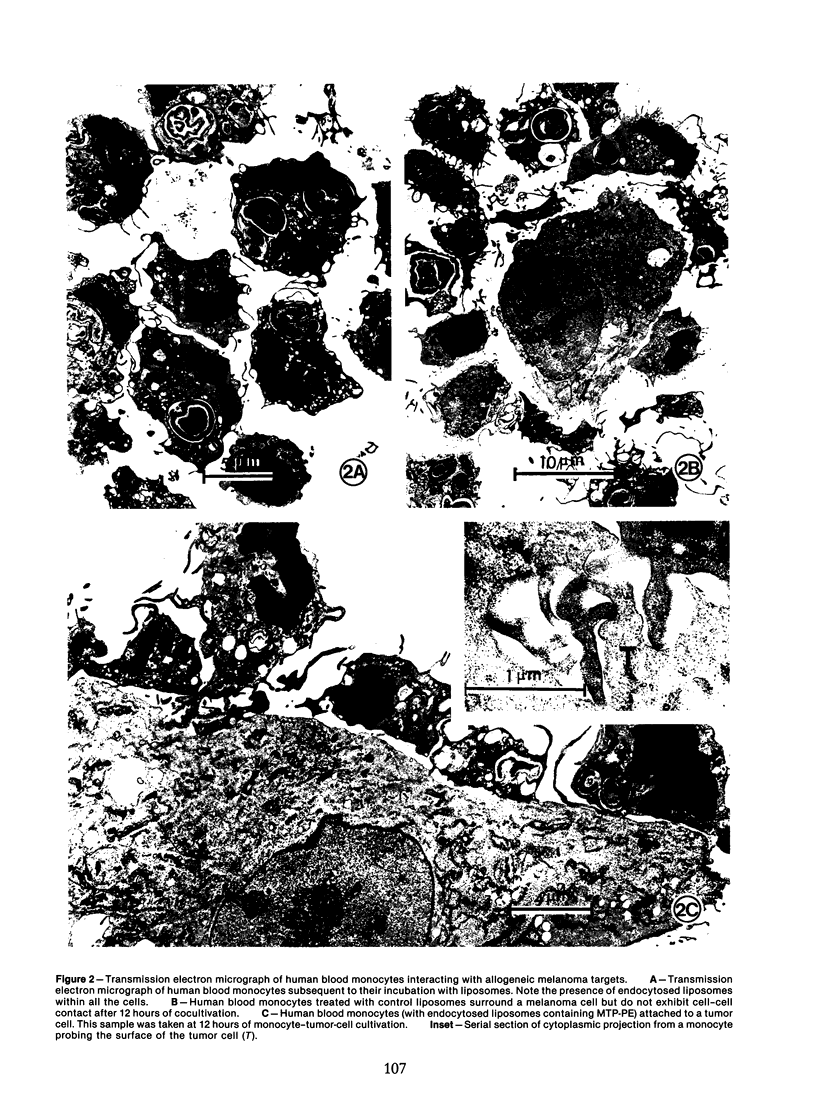
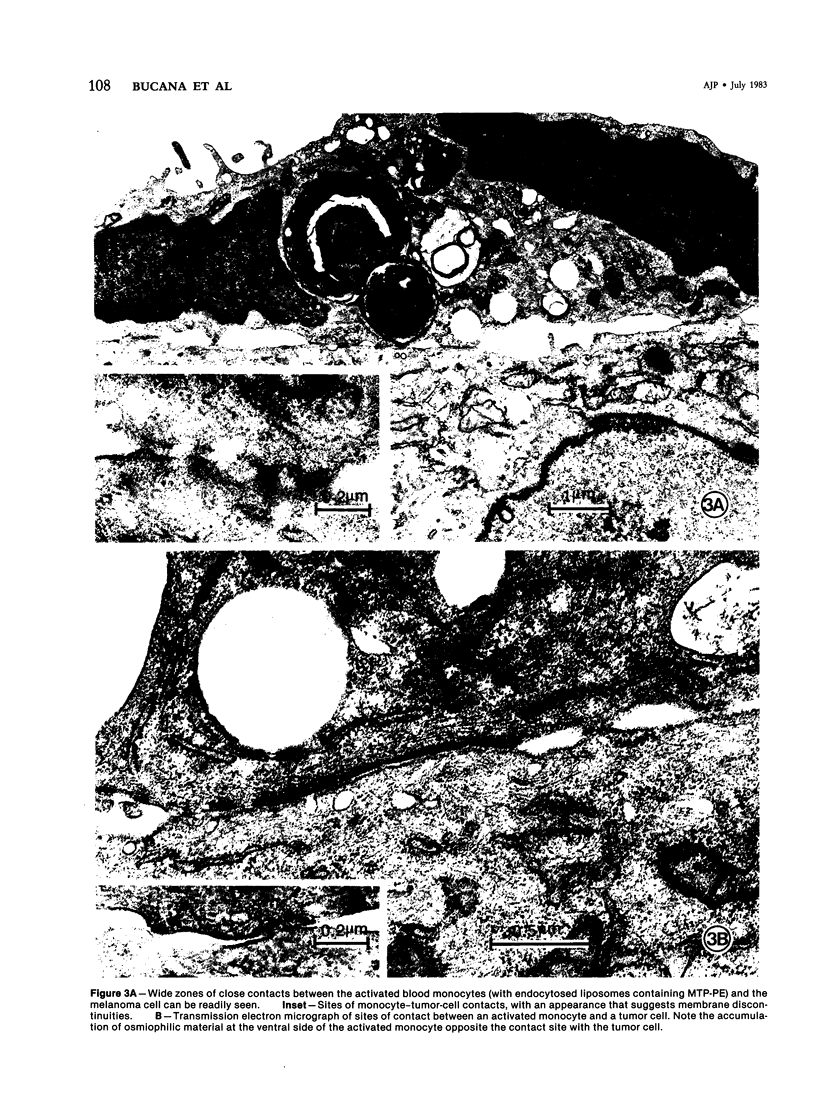
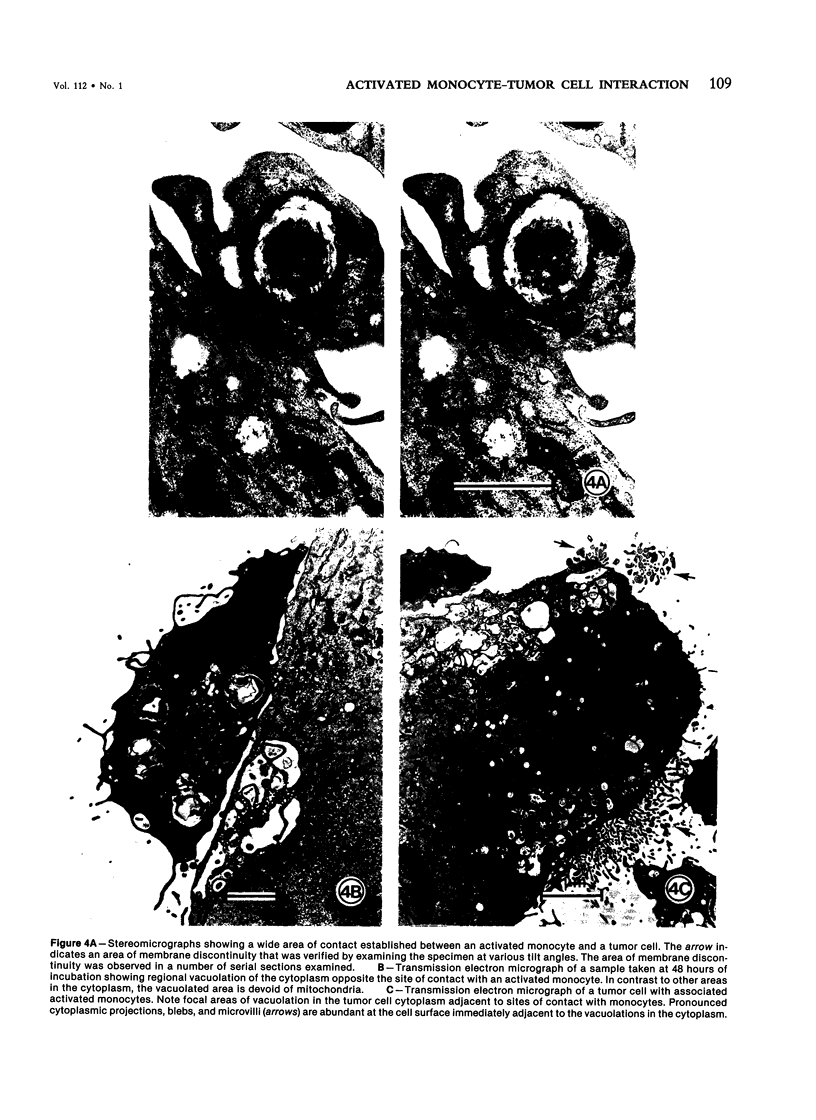
Human blood monocytes were activated to become tumoricidal by incubation with liposomes containing muramyl tripeptide-phosphatidylethanolamine, a lipophilic derivative of muramyl dipeptide. The interaction of both tumoricidal and control monocytes with target melanoma cells was analyzed by means of light microscopy and scanning and transmission electron microscopy. The authors found increased clustering around the melanoma cells by tumoricidal monocytes as compared with the control monocytes. The initial clustering of the tumoricidal monocytes around the tumor cells was followed by the establishment of numerous focal points of contact (binding), some of which actually exhibited areas of discontinuous membrane, a finding confirmed by stereophotography. By 24-48 hours of cocultivation, many of the target cells exhibited zones of vacuolation in the immediate vicinity of the tumoricidal monocytes, suggesting target cell damage. (This finding was confirmed by time-course cytotoxicity assays.) The authors conclude that tumor cell lysis mediated by activated human blood monocytes occurs as the final step in a process that includes the establishment of a direct cell-cell contact, damage to the target cell membrane, and the development of areas of vacuolation in the target cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Biesecker J. L., Koss L. G. The activation of mononuclear phagocytes in vitro: immunologically mediated enhancement. J Reticuloendothel Soc. 1973 Dec;14(6):550–570. [PubMed] [Google Scholar]
- Adams D. O. Effector mechanisms of cytolytically activated macrophages. I. Secretion of neutral proteases and effect of protease inhibitors. J Immunol. 1980 Jan;124(1):286–292. [PubMed] [Google Scholar]
- Aksamit R. R., Kim K. J. Macrophage cell lines produce a cytotoxin. J Immunol. 1979 May;122(5):1785–1790. [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bell R. B., McIvor K. L. The effects of a macrophage-derived cytotoxin on the growth and metabolism of target cells. Cell Immunol. 1976 Mar 1;22(1):19–27. doi: 10.1016/0008-8749(76)90003-4. [DOI] [PubMed] [Google Scholar]
- Boyle M. D., Ormerod M. G. The destruction of allogeneic tumour cells by peritoneal macrophages from immune mice: purification of lytic effector cells. Cell Immunol. 1975 May;17(1):247–258. doi: 10.1016/s0008-8749(75)80024-4. [DOI] [PubMed] [Google Scholar]
- Bucana C., Hoyer L. C., Hobbs B., Breesman S., McDaniel M., Hanna M. G., Jr Morphological evidence for the translocation of lysosomal organelles from cytotoxic macrophages into the cytoplasm of tumor target cells. Cancer Res. 1976 Dec;36(12):4444–4458. [PubMed] [Google Scholar]
- Cameron D. J., Churchill W. H. Cytotoxicity of human macrophages for tumor cells. Enhancement by human lymphocyte mediators. J Clin Invest. 1979 May;63(5):977–984. doi: 10.1172/JCI109398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers V. C., Weiser R. S. The ultrastructure of sarcoma I cells and immune macrophages during their interaction in the peritoneal cavities of immune C57BL-6 mice. Cancer Res. 1972 Feb;32(2):413–419. [PubMed] [Google Scholar]
- Currie G. A., Basham C. Activated macrophages release a factor which lyses malignant cells but not normal cells. J Exp Med. 1975 Dec 1;142(6):1600–1605. doi: 10.1084/jem.142.6.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A., Basham C. Differential arginine dependence and the selective cytotoxic effects of activated macrophages for malignant cells in vitro. Br J Cancer. 1978 Dec;38(6):653–659. doi: 10.1038/bjc.1978.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans K. P., Pels E., Den Otter W. Destruction of murine lymphoma cells by allogeneic immune peritoneal macrophages in vitro: an ultrastructure study. J Natl Cancer Inst. 1981 Jan;66(1):67–79. [PubMed] [Google Scholar]
- Ferluga J., Schorlemmer H. U., Baptista L. C., Allison A. C. Production of the complement cleavage product, C3a, by activated macrophages and its tumorolytic effects. Clin Exp Immunol. 1978 Mar;31(3):512–517. [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J. Recognition and destruction of target cells by tumoricidal macrophages. Isr J Med Sci. 1978 Jan;14(1):177–191. [PubMed] [Google Scholar]
- Gallily R., Ben-Ishay Z. Immune cytolysis of mouse macrophages in vitro. J Reticuloendothel Soc. 1975 Jul;18(1):44–52. [PubMed] [Google Scholar]
- Gmelig-Meyling F., Waldmann T. A. Separation of human blood monocytes and lymphocytes on a continuous Percoll gradient. J Immunol Methods. 1980;33(1):1–9. doi: 10.1016/0022-1759(80)90077-0. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr Discrimination between neoplastic and non-neoplastic cells in vitro by activated macrophages. J Natl Cancer Inst. 1974 Nov;53(5):1487–1492. doi: 10.1093/jnci/53.5.1487. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr Heterocytolysis by macrophages activated by bacillus Calmette-Guérin: lysosome exocytosis into tumor cells. Science. 1974 Apr 26;184(4135):468–471. doi: 10.1126/science.184.4135.468. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Resistance to murine tumors conferred by chronic infection with intracellular protozoa, Toxoplasma gondii and Besnoitia jellisoni. J Infect Dis. 1971 Dec;124(6):587–592. doi: 10.1093/infdis/124.6.587. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. J., Granger G. A. The in vitro induction and release of a cell toxin by immune C57B1-6 mouse peritoneal macrophages. Cell Immunol. 1972 Jan;3(1):88–100. doi: 10.1016/0008-8749(72)90229-8. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Dean J. H., Jerrells T. R., Herberman R. B. Augmentation of tumoricidal activity of human monocytes and macrophages by lymphokines. Int J Cancer. 1980 Jun 15;25(6):691–699. doi: 10.1002/ijc.2910250602. [DOI] [PubMed] [Google Scholar]
- Marino P. A., Adams D. O. Interaction of Bacillus Calmette--Guérin-activated macrophages and neoplastic cells in vitro II. The relationship of selective binding to cytolysis. Cell Immunol. 1980 Aug 15;54(1):26–35. doi: 10.1016/0008-8749(80)90186-0. [DOI] [PubMed] [Google Scholar]
- Marino P. A., Adams D. O. Interaction of Bacillus Calmette--Guérin-activated macrophages and neoplastic cells in vitro. I. Conditions of binding and its selectivity. Cell Immunol. 1980 Aug 15;54(1):11–25. doi: 10.1016/0008-8749(80)90185-9. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Brukner L. H., Silverstein S. C., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. I. Pharmacologic triggering of effector cells and the release of hydrogen peroxide. J Exp Med. 1979 Jan 1;149(1):84–99. doi: 10.1084/jem.149.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack G. R., Johnson R. J., Shin H. S. Tumor cell cytostasis by macrophages and antibody in vitro. I. Resolution into contact-dependent and contact-independent steps. J Immunol. 1978 May;120(5):1560–1566. [PubMed] [Google Scholar]
- Puvion F., Fray A., Halpern B. A cytochemical study of the in vitro interaction between normal and activated mouse peritoneal macrophages and tumor cells. J Ultrastruct Res. 1976 Jan;54(1):95–108. doi: 10.1016/s0022-5320(76)80012-3. [DOI] [PubMed] [Google Scholar]
- Schroit A. J., Fidler I. J. Effects of liposome structure and lipid composition on the activation of the tumoricidal properties of macrophages by liposomes containing muramyl dipeptide. Cancer Res. 1982 Jan;42(1):161–167. [PubMed] [Google Scholar]
- Snodgrass M. J., Hanna M. G., Jr Ultrastructural studies of histiocyte-tumor cell interactions during tumor regression after intralesional injection of Mycobacterium bovis. Cancer Res. 1973 Apr;33(4):701–716. [PubMed] [Google Scholar]
- Tsuruo T., Fidler I. J. Differences in drug sensitivity among tumor cells from parental tumors, selected variants, and spontaneous metastases. Cancer Res. 1981 Aug;41(8):3058–3064. [PubMed] [Google Scholar]
- Zucker-Franklin D., Lavie G., Franklin E. C. Demonstration of membrane-bound proteolytic activity on the surface of mononuclear leukocytes. J Histochem Cytochem. 1981 Mar;29(3A):451–456. doi: 10.1177/29.3.451. [DOI] [PubMed] [Google Scholar]