Abstract
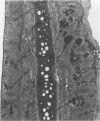
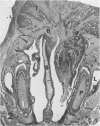
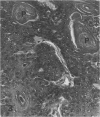
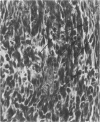
Charles River-CD Sprague-Dawley rats in 3 equal groups of 100 males and 100 females each were exposed to 12, 1, and 0 ppm of phenylglycidyl ether vapor for 24 months. Nasal tumors were first detected after 621 days' exposure at 12 ppm with an incidence of 11% in males and 4.4% in females. No nasal tumors were found at 1 ppm in rats exposed for 24 months. The nasal tumors, mostly epidermoid carcinomas, were derived from the respiratory epithelium and nasal glands, both of which revealed squamous metaplasia or dysplasia in the anterior nasal cavity. Most nasal tumors were confined to the anterior nasal cavity and occasionally invaded the dorsonasal bones and posterior nasal cavity. The undifferentiated glandular cells appear to differentiate to neoplastic squamous cells, because the ultrastructure of epidermoid carcinoma revealed traits of glandular cell differentiation in the neoplastic squamous cells. The features of glandular cell differentiation in the neoplastic squamous cells were intercellular or intracellular glandular lumens, secretory vesicles, mucus droplets, and intermediate cells showing both glandular and squamous differentiation. Squamous cells in the well-differentiated epidermoid carcinomas revealed abundant tonofibrils, desmosomes, glycogen particulates, and interdigitated cytoplasmic processes. These markers of squamous-cell differentiation were markedly reduced in the undifferentiated epidermoid carcinomas. The spindle-cell squamous carcinoma showed both squamous and fibroblastic-like differentiations. Some spindle cells had only fibroblastic-like differentiation, suggesting spindle-cell metaplasia of the squamous cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELMAN H. D., OBERMAN H. A. SQUAMOUS CELL CARCINOMA OF THE LARYNX WITH SARCOMA-LIKE STROMA. A CLINICOPATHOLOGIC ASSESSMENT OF SPINDLE CELL CARCINOMA AND "PSEUDOSARCOMA". Am J Clin Pathol. 1965 Aug;44:135–145. doi: 10.1093/ajcp/44.2.135. [DOI] [PubMed] [Google Scholar]
- APPELMAN H. D., OBERMAN H. A. SQUAMOUS CELL CARCINOMA OF THE LARYNX WITH SARCOMA-LIKE STROMA. A CLINICOPATHOLOGIC ASSESSMENT OF SPINDLE CELL CARCINOMA AND "PSEUDOSARCOMA". Am J Clin Pathol. 1965 Aug;44:135–145. doi: 10.1093/ajcp/44.2.135. [DOI] [PubMed] [Google Scholar]
- Acheson E. D., Cowdell R. H., Jolles B. Nasal cancer in the Northamptonshire boot and shoe industry. Br Med J. 1970 Feb 14;1(5693):385–393. doi: 10.1136/bmj.1.5693.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson E. D. Nasal cancer in the furniture and boot and shoe manufacturing industries. Prev Med. 1976 Jun;5(2):295–315. doi: 10.1016/0091-7435(76)90046-3. [DOI] [PubMed] [Google Scholar]
- Auersperg N., Erber H. Effects of tissue culture environment on growth patterns and ultrastructure of poorly differentiated human cervical carcinomas. J Natl Cancer Inst. 1976 Nov;57(5):981–994. doi: 10.1093/jnci/57.5.981. [DOI] [PubMed] [Google Scholar]
- Battifora H. Spindle cell carcinoma: ultrastructural evidence of squamous origin and collagen production by the tumor cells. Cancer. 1976 May;37(5):2275–2282. doi: 10.1002/1097-0142(197605)37:5<2275::aid-cncr2820370518>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Boysen M., Reith A. Intracytoplasmic lumina with and without cilia in both normal and pathologically altered nasal mucosa. Ultrastruct Pathol. 1980 Oct-Dec;1(4):477–485. doi: 10.3109/01913128009140554. [DOI] [PubMed] [Google Scholar]
- Cecchi F., Buiatti E., Kriebel D., Nastasi L., Santucci M. Adenocarcinoma of the nose and paranasal sinuses in shoemakers and woodworkers in the province of Florence, Italy (1963-77). Br J Ind Med. 1980 Aug;37(3):222–225. doi: 10.1136/oem.37.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Boulay C. E., Isaacson P. Carcinoma of the oesophagus with spindle cell features. Histopathology. 1981 Jul;5(4):403–414. doi: 10.1111/j.1365-2559.1981.tb01801.x. [DOI] [PubMed] [Google Scholar]
- Erlandson R. A., Carstens P. H. Ultrastructure of tubular carcinoma of the breast. Cancer. 1972 Apr;29(4):987–995. doi: 10.1002/1097-0142(197204)29:4<987::aid-cncr2820290446>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Gelfman W. E., Williams A. Spindle-cell carcinoma of the tongue. Report of a case. Oral Surg Oral Med Oral Pathol. 1969 May;27(5):659–663. doi: 10.1016/0030-4220(69)90102-9. [DOI] [PubMed] [Google Scholar]
- Hadfield E. H. Cancer hazard from wood dust and in the boot and shoe industry. Ann Occup Hyg. 1972 Apr;15(1):39–41. doi: 10.1093/annhyg/15.1.39. [DOI] [PubMed] [Google Scholar]
- Hadfield E. H., Macbeth R. G. Adenocarcinoma of ethmoids in furniture workers. Ann Otol Rhinol Laryngol. 1971 Oct;80(5):699–703. doi: 10.1177/000348947108000512. [DOI] [PubMed] [Google Scholar]
- Hall-Craggs M., Toker C., Nedwich A. Carcinosarcoma of the uterine cervix: a light and electron microscopic study. Cancer. 1981 Jul 1;48(1):161–169. doi: 10.1002/1097-0142(19810701)48:1<161::aid-cncr2820480127>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Klacsmann P. G., Olson J. L., Eggleston J. C. Mucoepidermoid carcinoma of the bronchus: an electron microscopic study of the low grade and the high grade variants. Cancer. 1979 May;43(5):1720–1733. doi: 10.1002/1097-0142(197905)43:5<1720::aid-cncr2820430523>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Klacsmann P. G., Olson J. L., Eggleston J. C. Mucoepidermoid carcinoma of the bronchus: an electron microscopic study of the low grade and the high grade variants. Cancer. 1979 May;43(5):1720–1733. doi: 10.1002/1097-0142(197905)43:5<1720::aid-cncr2820430523>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Konetzke G. W. Die kanzerogene Wirkung von Arsen und Nickel. Arch Geschwulstforsch. 1974;44(1):16–22. [PubMed] [Google Scholar]
- Kuschner M., Laskin S., Drew R. T., Cappiello V., Nelson N. Inhalation carcinogenicity of alpha halo ethers. III. Lifetime and limited period inhalation studies with bis(chloromethyl)ether at 0.1 ppm. Arch Environ Health. 1975 Feb;30(2):73–77. doi: 10.1080/00039896.1975.10666646. [DOI] [PubMed] [Google Scholar]
- LEAKE D., EVANS R. E., WEISBERGER D. SQUAMOUS CELL CARCINOMA ASSOCIATED WITH PSEUDOSARCOMA: REPORT OF TWO CASES. J Oral Surg. 1965 Jul;23:446–450. [PubMed] [Google Scholar]
- Laskin S., Drew R. T., Capiello V., Kuschner M., Nelson N. Inhalation carcinogenicity of alpha halo ethers. II. Chronic inhalation studies with chloromethyl methyl ether. Arch Environ Health. 1975 Feb;30(2):70–72. doi: 10.1080/00039896.1975.10666645. [DOI] [PubMed] [Google Scholar]
- Laskin S., Kuschner M., Drew R. T., Cappiello V. P., Nelson N. Tumors of the respiratory tract induced by inhalation of bis(chloromethyl)ether. Arch Environ Health. 1971 Aug;23(2):135–136. doi: 10.1080/00039896.1971.10665970. [DOI] [PubMed] [Google Scholar]
- Lee K. P., Trochimowicz H. J. Induction of nasal tumors in rats exposed to hexamethylphosphoramide by inhalation. J Natl Cancer Inst. 1982 Jan;68(1):157–171. [PubMed] [Google Scholar]
- Lichtiger B., Mackay B., Tessmer C. F. Spindle-cell variant of squamous carcinoma. A light and electron microscopic study of 13 cases. Cancer. 1970 Dec;26(6):1311–1320. doi: 10.1002/1097-0142(197012)26:6<1311::aid-cncr2820260618>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- MITTELMAN G. J., PICKLE D. E., SCOPP I. W., GREENE G. W., Jr SPINDLE-CELL CARCINOMA OF THE FLOOR OF THE MOUTH; REPORT OF A CASE. Oral Surg Oral Med Oral Pathol. 1965 Sep;20:399–406. doi: 10.1016/0030-4220(65)90174-x. [DOI] [PubMed] [Google Scholar]
- Martin M. R., Kahn L. B. So-called pseudosarcoma of the esophagus: nodal metastases of the spindle cell element. Arch Pathol Lab Med. 1977 Nov;101(11):604–609. [PubMed] [Google Scholar]
- Mosbech J., Acheson E. D. Nasal cancer in furniture-makers in Denmark. Dan Med Bull. 1971 May;18(2):34–35. [PubMed] [Google Scholar]
- Patterson S. D., Ballard R. W. Nasal blastoma: a light and electron microscopic study. Ultrastruct Pathol. 1980 Oct-Dec;1(4):487–494. doi: 10.3109/01913128009140555. [DOI] [PubMed] [Google Scholar]
- Pedersen E., Hogetveit A. C., Andersen A. Cancer of respiratory organs among workers at a nickel refinery in Norway. Int J Cancer. 1973 Jul 15;12(1):32–41. doi: 10.1002/ijc.2910120104. [DOI] [PubMed] [Google Scholar]
- Swenberg J. A., Kerns W. D., Mitchell R. I., Gralla E. J., Pavkov K. L. Induction of squamous cell carcinomas of the rat nasal cavity by inhalation exposure to formaldehyde vapor. Cancer Res. 1980 Sep;40(9):3398–3402. [PubMed] [Google Scholar]
- Tomita T., Lotuaco L., Talbott L., Watanabe I. Mucoepidermoid carcinoma of the subglottis. An ultrastructural study. Arch Pathol Lab Med. 1977 Mar;101(3):145–148. [PubMed] [Google Scholar]
- Trump B. F., McDowell E. M., Glavin F., Barrett L. A., Becci P. J., Schürch W., Kaiser H. E., Harris C. C. The respiratory epithelium. III. Histogenesis of epidermoid metaplasia and carcinoma in situ in the human. J Natl Cancer Inst. 1978 Aug;61(2):563–575. [PubMed] [Google Scholar]
- Wang N. S., Huang S. N., Thurlbeck W. M. Squamous metaplasia of the opening of bronchial glands. Am J Pathol. 1972 Jun;67(3):571–582. [PMC free article] [PubMed] [Google Scholar]
- Woyke S., Domagala W., Olszewski W., Korabiec M. Pseudosarcoma of the skin. An electron microscopic study and comparison with the fine structure of spindle-cell variant of squamous carcinoma. Cancer. 1974 Apr;33(4):970–980. doi: 10.1002/1097-0142(197404)33:4<970::aid-cncr2820330412>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]