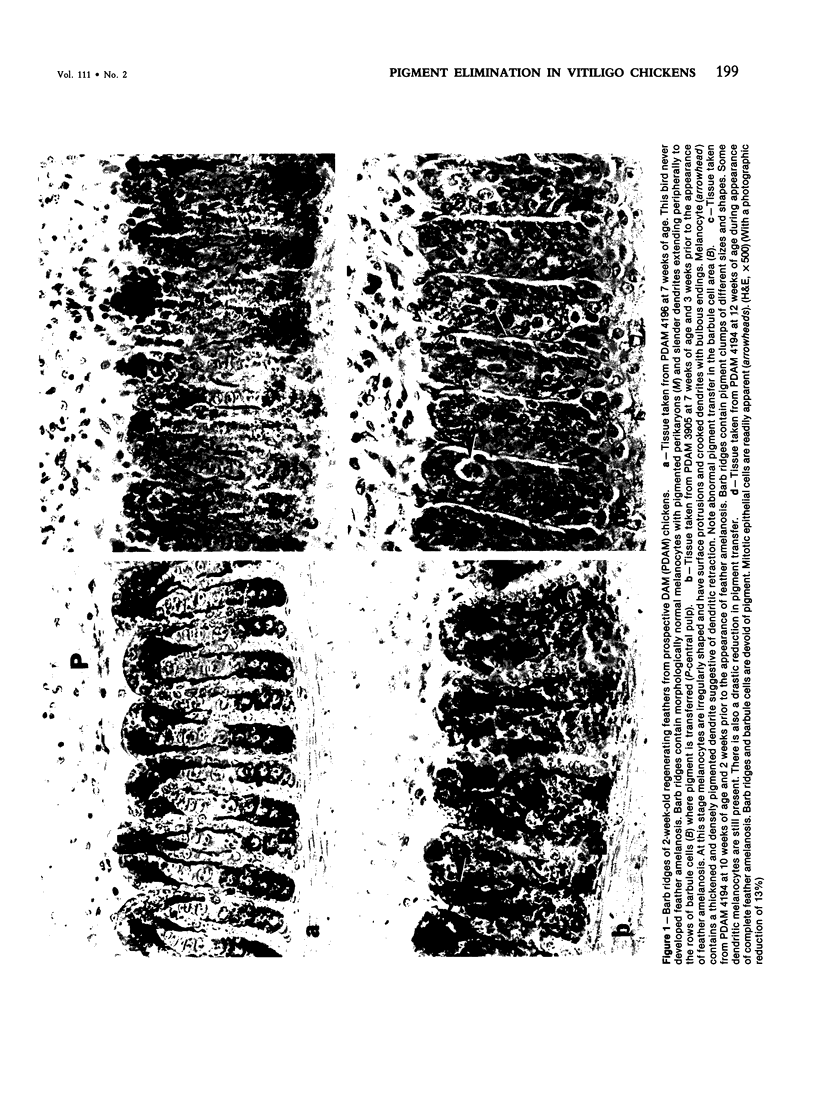
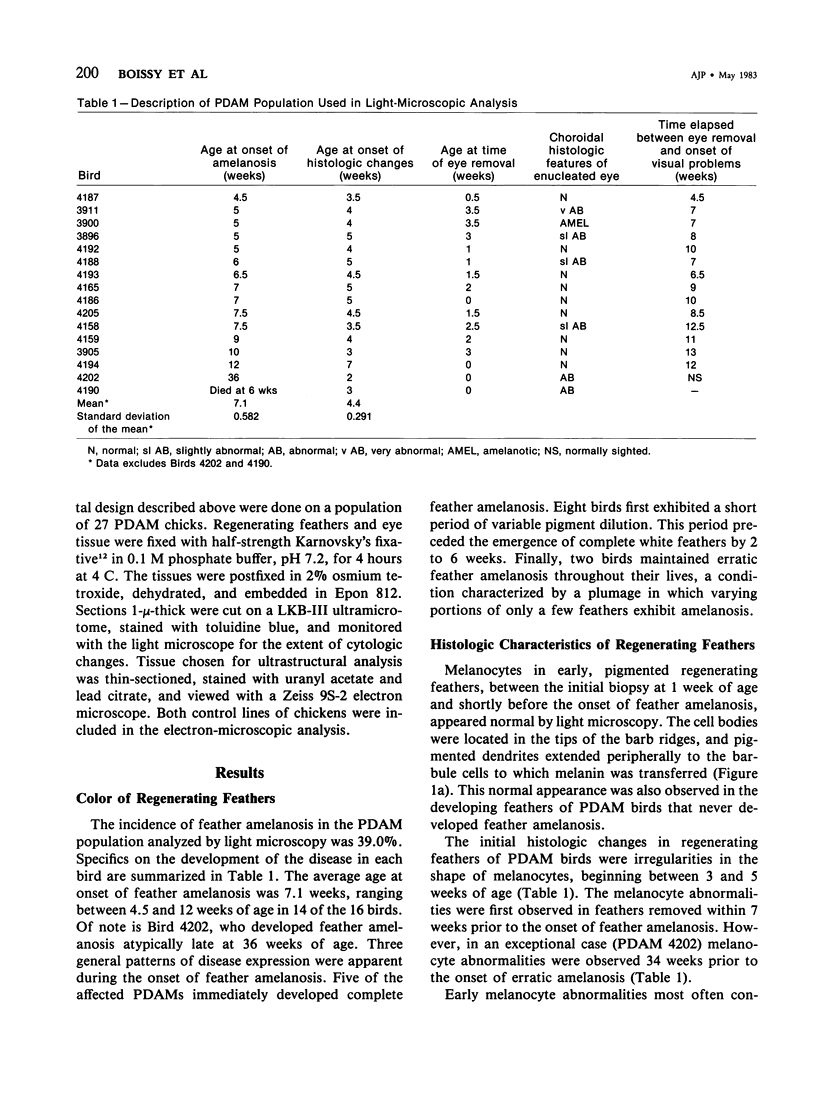
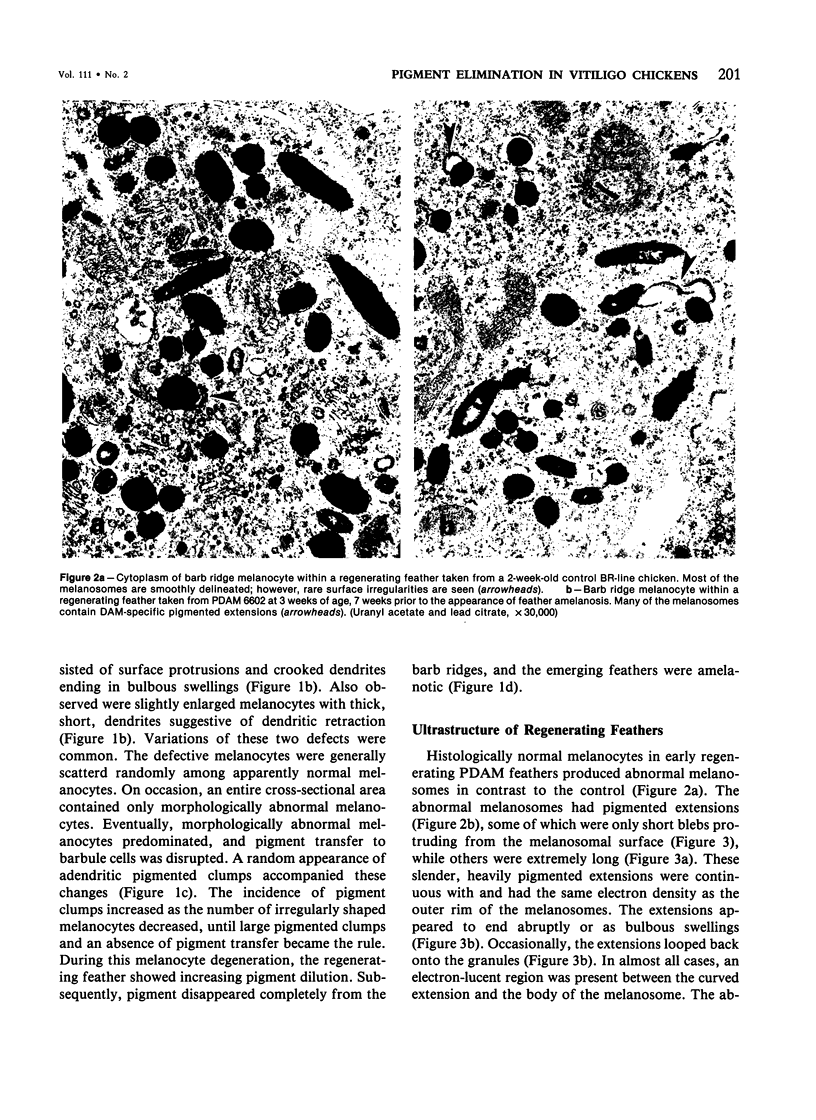
Abstract
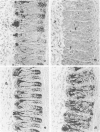
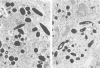
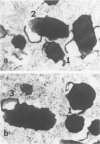
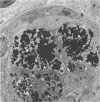
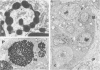
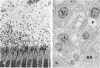
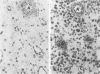
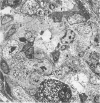
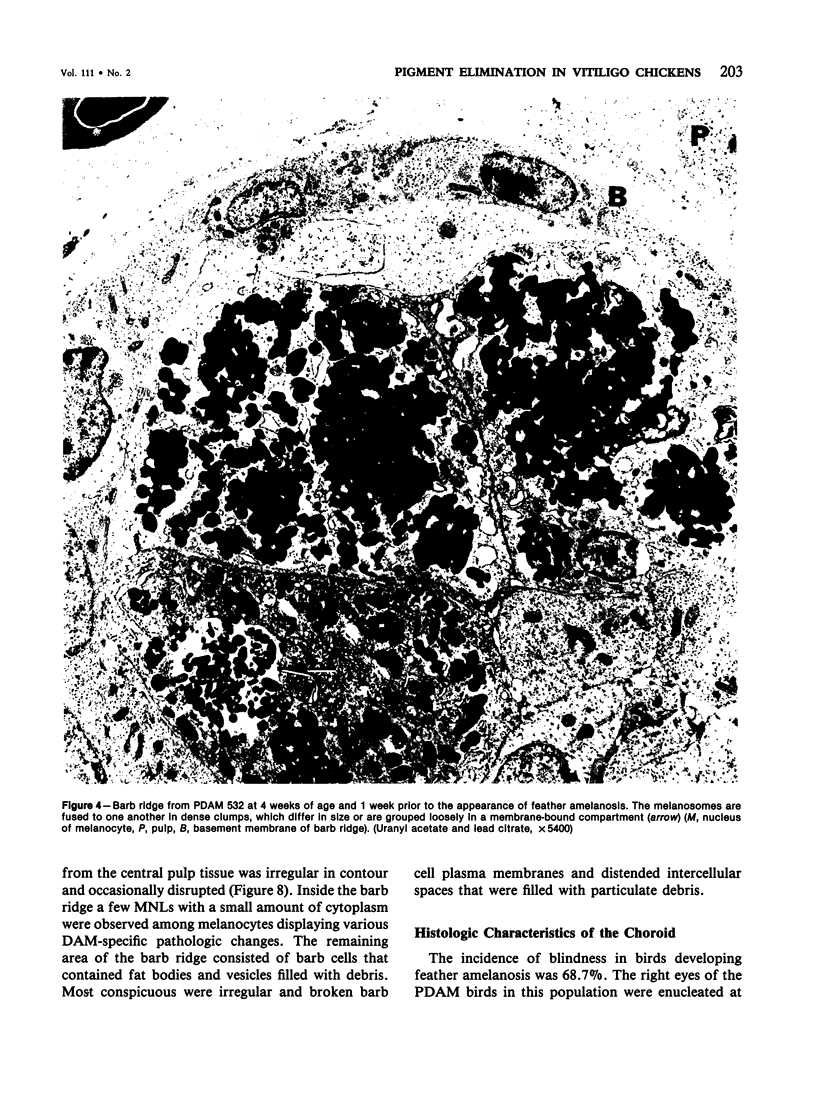
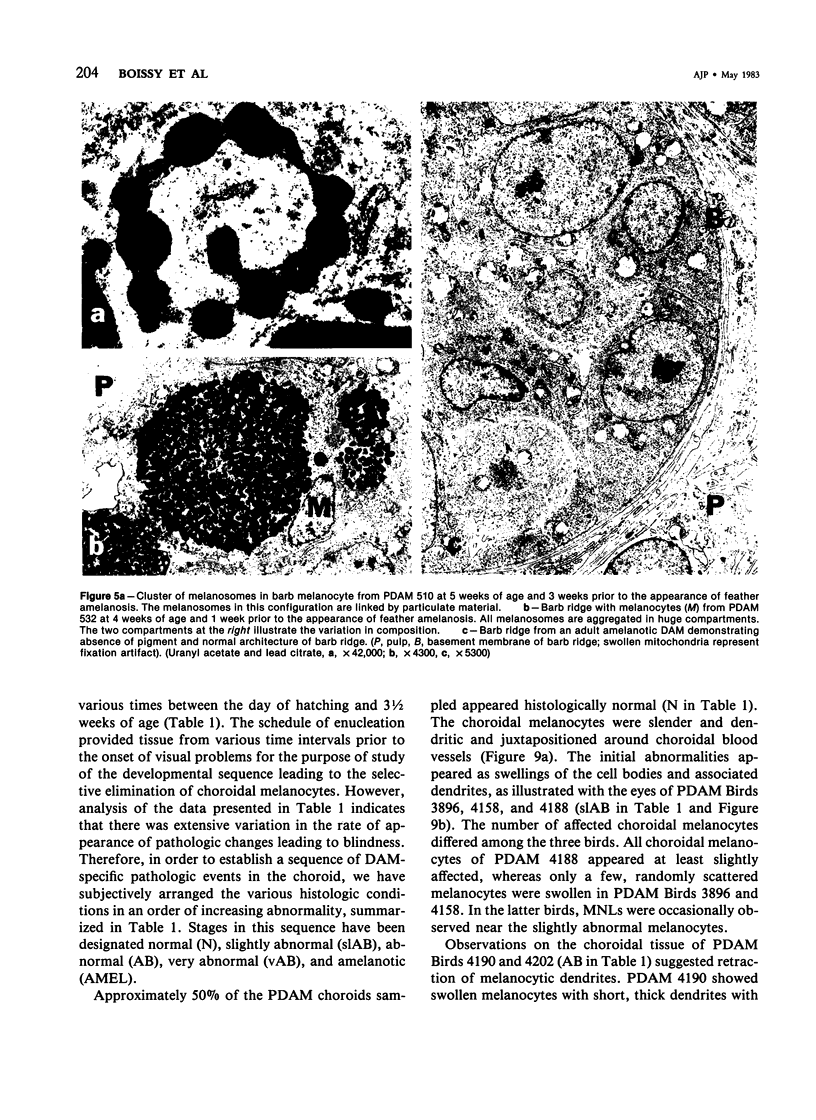
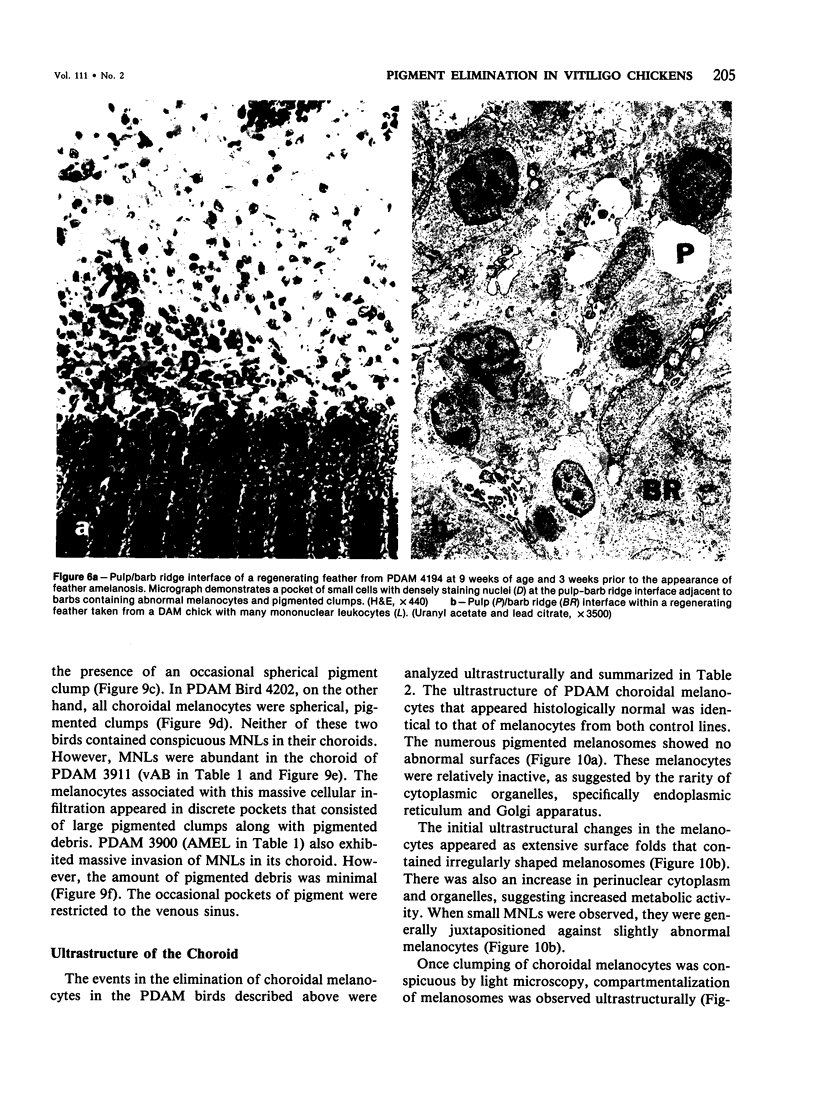
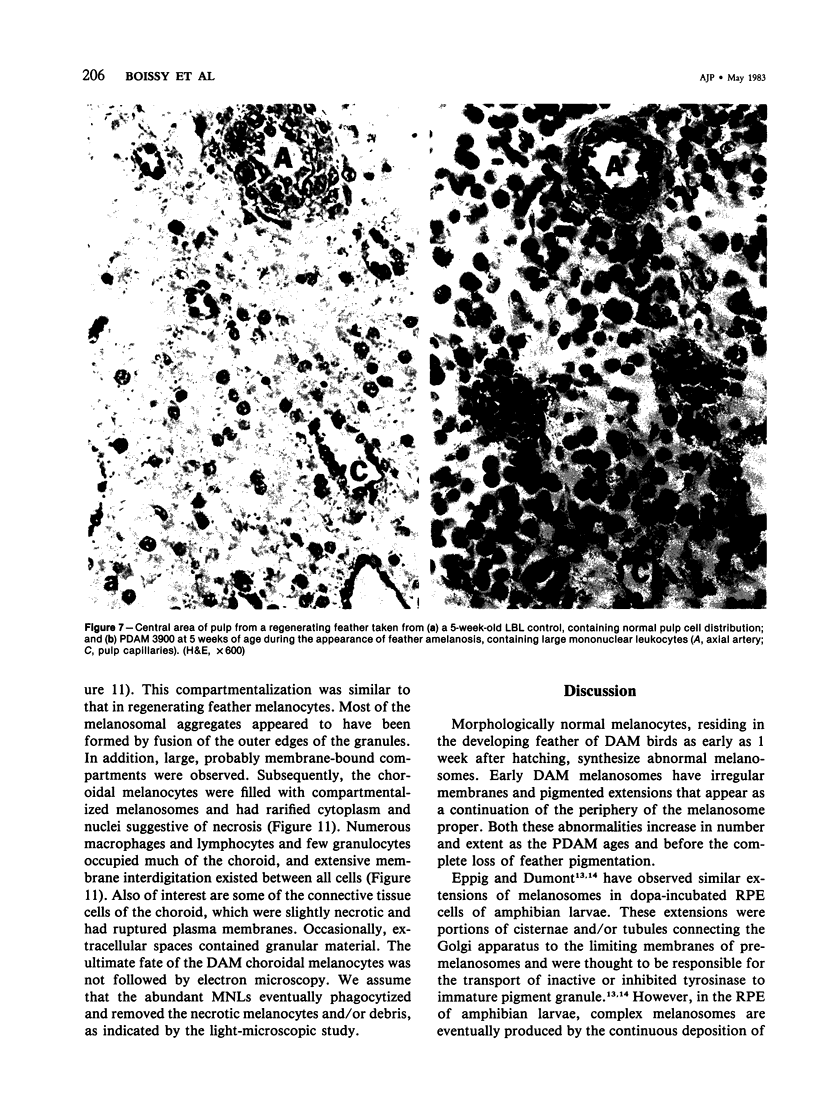
Newly hatched Gallus domesticus chicks of the delayed amelanotic (DAM) line have phenotypically normal down pigmentation. Functioning pigment cells are present in the down plumage, choroid, and retinal pigment epithelium. However, histologic and ultrastructural studies reveal that after hatching regenerating feather melanocytes synthesize melanosomes with abnormal, irregularly shaped surfaces and pigmented extensions. Eventually retraction of melanocytic dendrites and clumping of pigment occurs concomitantly with intracellular compartmentalization of the abnormal melanosomes. Melanocyte degeneration is accompanied by the appearance of mononuclear leukocytes (MNLs) in the pulp of the regenerating feathers. Concurrently, melanocytes cease to migrate into the regenerating feather epithelium, and the result is amelanosis. Changes in choroidal melanocytes are first evident as swelling of cell bodies and associated dendrites. Ultrastructurally, the choroidal melanocytes demonstrate increased cytoplasmic material, melanosomal irregularities, retraction of dendrites, melanosome compartmentalization, and eventual necrosis. Concurrently, MNLs arrive and remove the pigment from the choroid. The authors conclude that a basic melanocyte defect precedes the arrival of immunocytes in the delayed cutaneous and choroidal amelanosis in the genetic DAM vitiligo model of the chicken.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert D. M., Nordlund J. J., Lerner A. B. Ocular abnormalities occurring with vitiligo. Ophthalmology. 1979 Jun;86(6):1145–1160. doi: 10.1016/s0161-6420(79)35413-6. [DOI] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Dumont J. N. Oogenesis in Xenopus laevis (Daudin). II. The induction and subcellular localization of tyrosinase activity in developing oocytes. Dev Biol. 1974 Feb;36(2):330–342. doi: 10.1016/0012-1606(74)90055-4. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Jr, Dumont J. N. Cytochemical localization of tyrosinase activity in pigmented epithelial cells of Rana pipiens and Xenopus laevis larvae. J Ultrastruct Res. 1972 May;39(3):397–410. doi: 10.1016/s0022-5320(72)90031-7. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Jr Melanogenesis in amphibians. 3. The buoyant density of oocyte and larval xenopus laevis melanosomes and the isolation of oocyte melanosomes from the eyes of PTU-treated larvae. J Exp Zool. 1970 Dec;175(4):467–475. doi: 10.1002/jez.1401750406. [DOI] [PubMed] [Google Scholar]
- Feeney-Burns L., Mixon R. N. Development of amelanotic retinal pigment epithelium in eyes with a tapetum lacidum: melanosome autophagy and termination of melanogenesis. Dev Biol. 1979 Sep;72(1):73–88. doi: 10.1016/0012-1606(79)90099-x. [DOI] [PubMed] [Google Scholar]
- HOCHSTEIN P., COHEN G. The cytotoxicity of melanin precursors. Ann N Y Acad Sci. 1963 Feb 15;100:876–886. [PubMed] [Google Scholar]
- Halaban R., Lerner A. B. Tyrosinase and inhibition of proliferation of melanoma cells and fibroblasts. Exp Cell Res. 1977 Aug;108(1):119–125. doi: 10.1016/s0014-4827(77)80017-7. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Dilute (d) coat colour mutation of DBA/2J mice is associated with the site of integration of an ecotropic MuLV genome. Nature. 1981 Oct 1;293(5831):370–374. doi: 10.1038/293370a0. [DOI] [PubMed] [Google Scholar]
- Jimbow K., Szabo G., Fitzpatrick T. B. Ultrastructural investigation of autophagocytosis of melanosomes and programmed death of melanocytes in White Leghorn feathers: a study of morphogenetic events leading to hypomelanosis. Dev Biol. 1974 Jan;36(1):8–23. doi: 10.1016/0012-1606(74)90187-0. [DOI] [PubMed] [Google Scholar]
- Lamont S. J., Boissy R. E., Smyth J. R., Jr Humoral immune response and expression of spontaneous postnatal amelanosis in DAM line chickens. Immunol Commun. 1982;11(2):121–127. doi: 10.3109/08820138209057748. [DOI] [PubMed] [Google Scholar]
- Lamont S. J., Smyth J. R., Jr Effect of bursectomy on development of a spontaneous postnatal amelanosis. Clin Immunol Immunopathol. 1981 Dec;21(3):407–411. doi: 10.1016/0090-1229(81)90229-4. [DOI] [PubMed] [Google Scholar]
- Lerner A. B. On the etiology of vitiligo and gray hair. Am J Med. 1971 Aug;51(2):141–147. doi: 10.1016/0002-9343(71)90232-4. [DOI] [PubMed] [Google Scholar]
- Maul G. G., Brumbaugh J. A. On the possible function of coated vesicles in melanogenesis of the regenerating fowl feather. J Cell Biol. 1971 Jan;48(1):41–48. doi: 10.1083/jcb.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G. Golgi-melanosome relationship in human melanoma in vitro. J Ultrastruct Res. 1969 Jan;26(1):163–176. doi: 10.1016/s0022-5320(69)90042-2. [DOI] [PubMed] [Google Scholar]
- Moellmann G., Klein-Angerer S., Scollay D. A., Nordlund J. J., Lerner A. B. Extracellular granular material and degeneration of keratinocytes in the normally pigmented epidermis of patients with vitiligo. J Invest Dermatol. 1982 Nov;79(5):321–330. doi: 10.1111/1523-1747.ep12500086. [DOI] [PubMed] [Google Scholar]
- Nordlund J. J., Lerner A. B. Vitiligo. It is important. Arch Dermatol. 1982 Jan;118(1):5–8. doi: 10.1001/archderm.118.1.5. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Albala A., Biempica L. Ultrastructural and cytochemical observations on B-16 and Harding-Passey mouse melanomas. The origin of premelanosomes and compound melanosomes. J Histochem Cytochem. 1968 May;16(5):299–319. doi: 10.1177/16.5.299. [DOI] [PubMed] [Google Scholar]
- Pawelek J., Körner A., Bergstrom A., Bologna J. New regulators of melanin biosynthesis and the autodestruction of melanoma cells. Nature. 1980 Aug 7;286(5773):617–619. doi: 10.1038/286617a0. [DOI] [PubMed] [Google Scholar]
- Smyth J. R., Jr, Boissy R. E., Fite K. V., Albert D. M. Retinal dystrophy associated with a postnatal amelanosis in the chicken. Invest Ophthalmol Vis Sci. 1981 Jun;20(6):799–803. [PubMed] [Google Scholar]
- Smyth J. R., Jr, Boissy R. E., Fite K. V. The DAM chicken: a model for spontaneous postnatal cutaneous and ocular amelanosis. J Hered. 1981 May-Jun;72(3):150–156. [PubMed] [Google Scholar]
- Whitney J. B., 3rd, Lamoreux M. L. Transposable elements controlling genetic instabilities in mammals. J Hered. 1982 Jan-Feb;73(1):12–18. doi: 10.1093/oxfordjournals.jhered.a109567. [DOI] [PubMed] [Google Scholar]