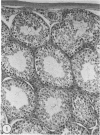
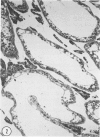
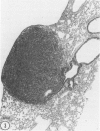
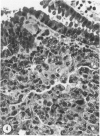
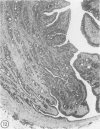
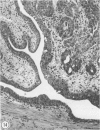
Abstract
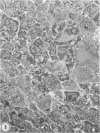
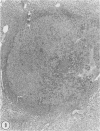
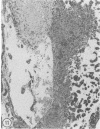
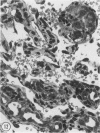
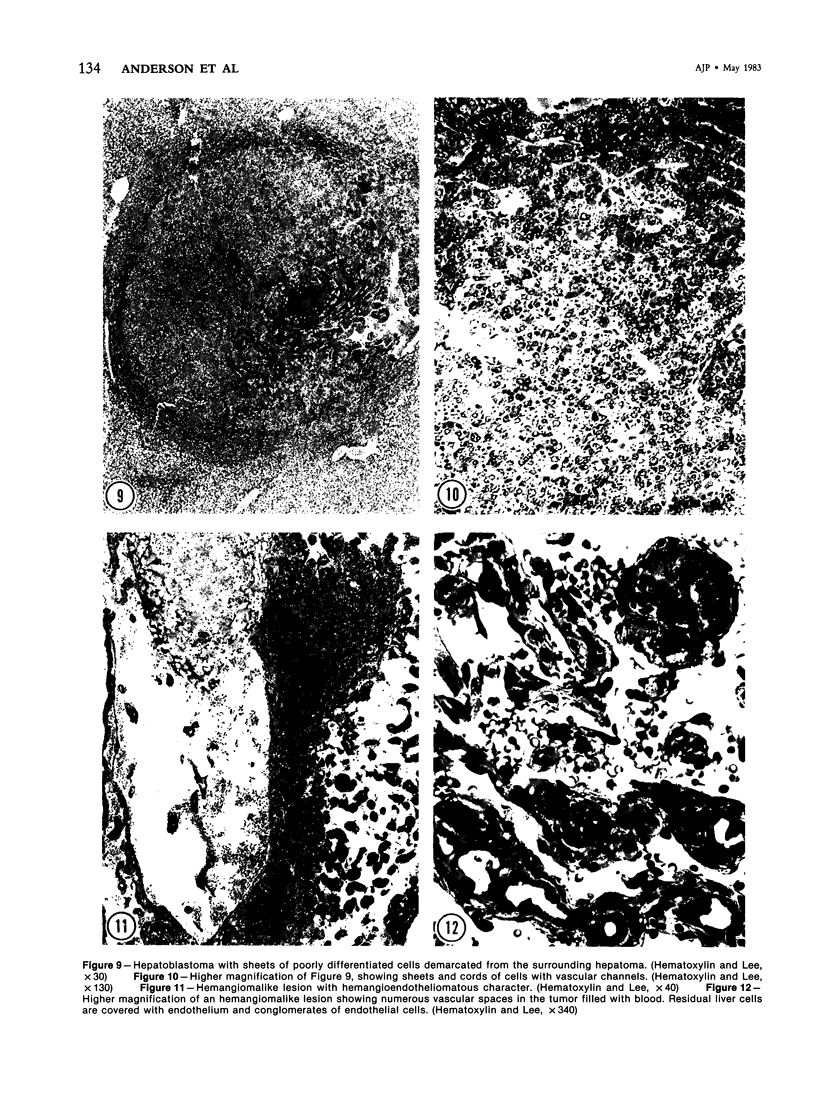
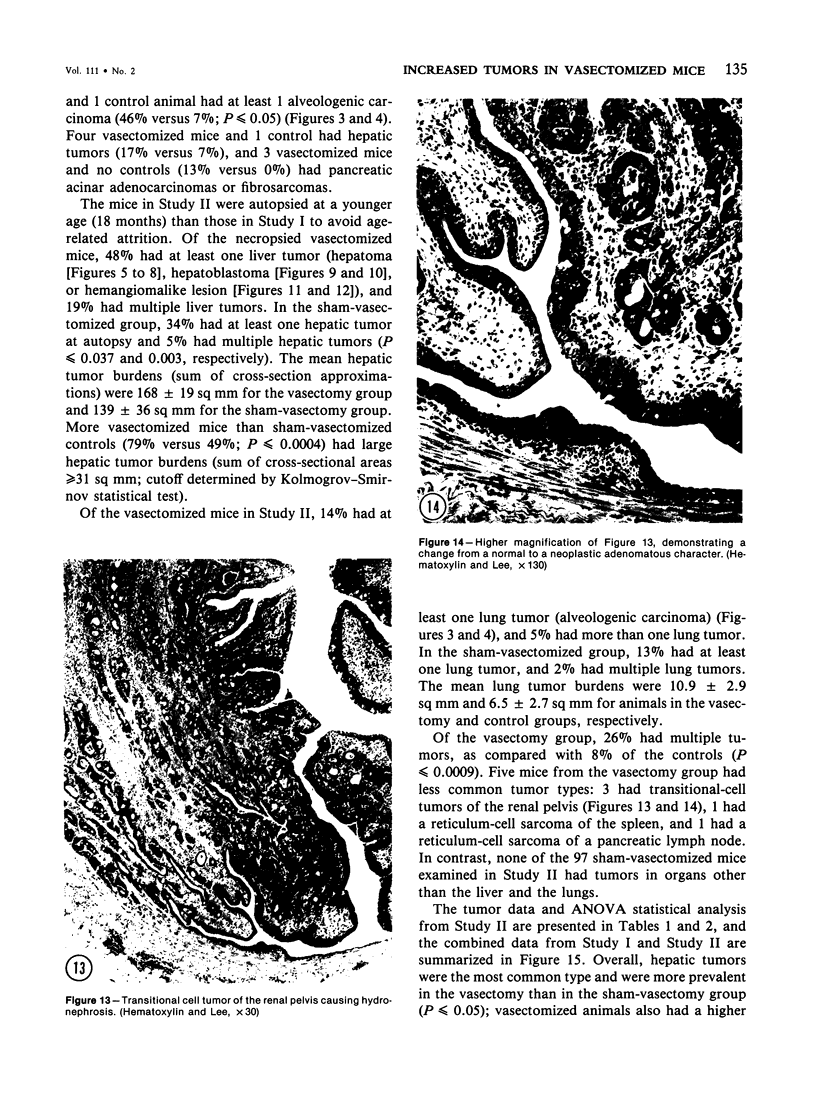
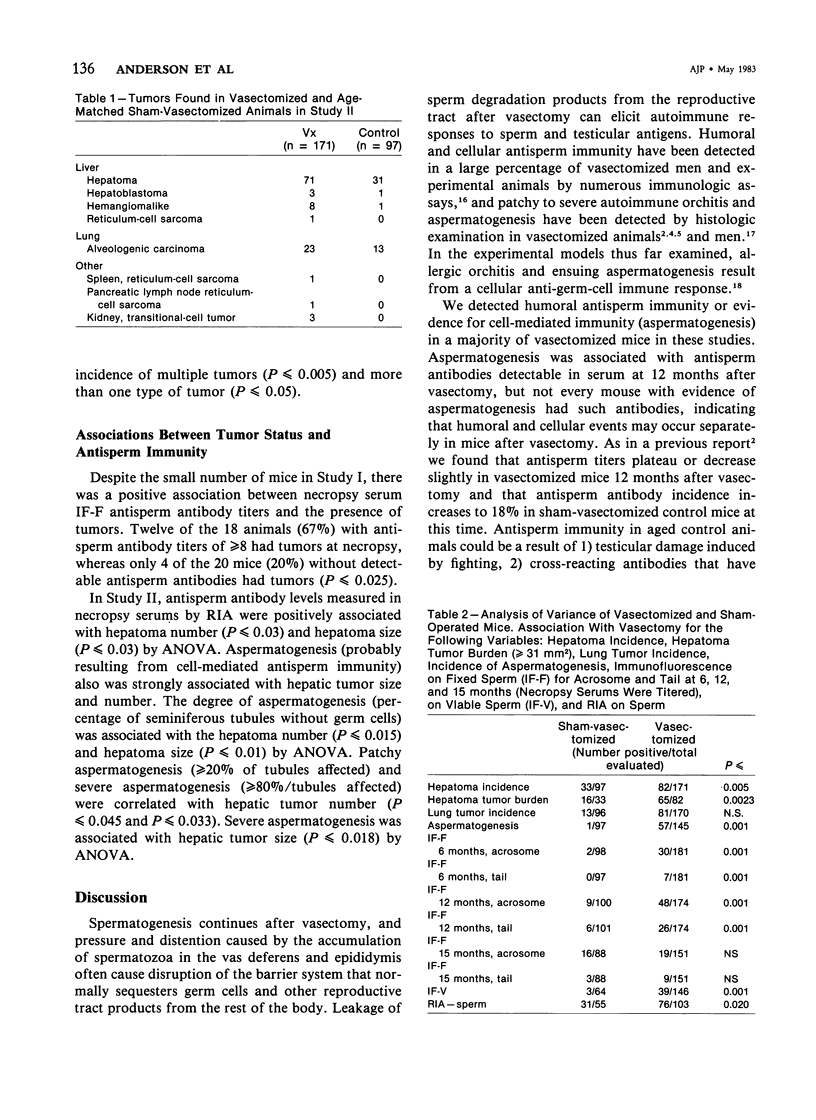
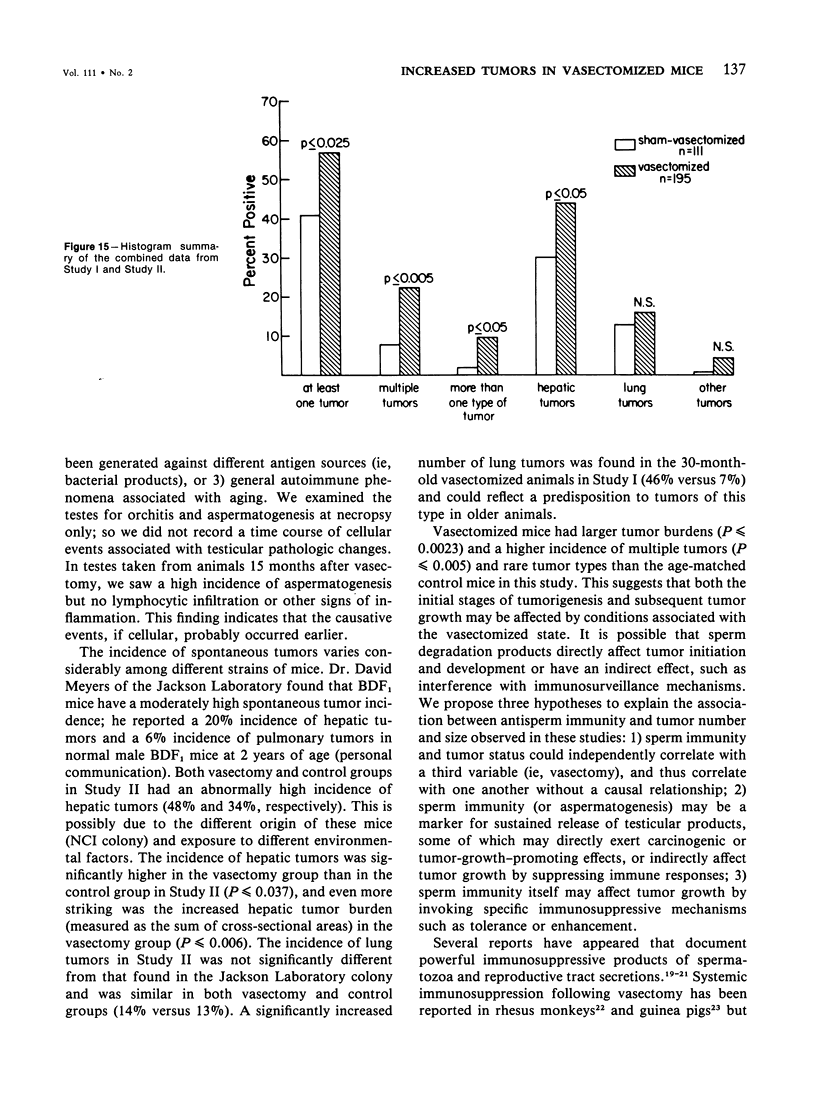
In two independent studies the authors have observed a significantly higher incidence of spontaneous tumors in vasectomized BDF1 mice over the long term than in age-matched sham-vasectomized control mice. In the first study, necropsies were performed on the animals at 30 months of age (27 months after surgery), and 15 of 24 vasectomized versus 2 of 14 sham-vasectomized mice (P less than or equal to 0.025) had detectable tumors in various tissues. In a second study, necropsies were performed on the animals at a younger age (18 months, 15 months after surgery), and liver tumors predominated: 82 of 171 vasectomized versus 33 of 97 controls (48% versus 34%, P less than or equal to 0.037) had at least one hepatic tumor, and a significantly higher percentage of vasectomized animals had large (greater than or equal to 31 sq mm) hepatic tumor burdens (80% versus 49%; P less than or equal to 0.002) and multiple hepatic tumors (19% versus 5%; P less than or equal to 0.002). In combined data from both studies, the vasectomy group had a higher incidence of (1) at least one tumor (P less than or equal to 0.025), (2) multiple tumors (P less than or equal to 0.005), and (3) more than one type of tumor (P less than or equal to 0.05. Furthermore, in both studies tumor number and size were significantly associated with antisperm immunity detected by antibody or aspermatogenesis evaluation. It is speculated that sperm degradation products and/or the autoimmune response to sperm that commonly accompanies vasectomy may affect tumor induction or growth directly or indirectly by interfering with immunosurveillance mechanisms.
Full text
PDF


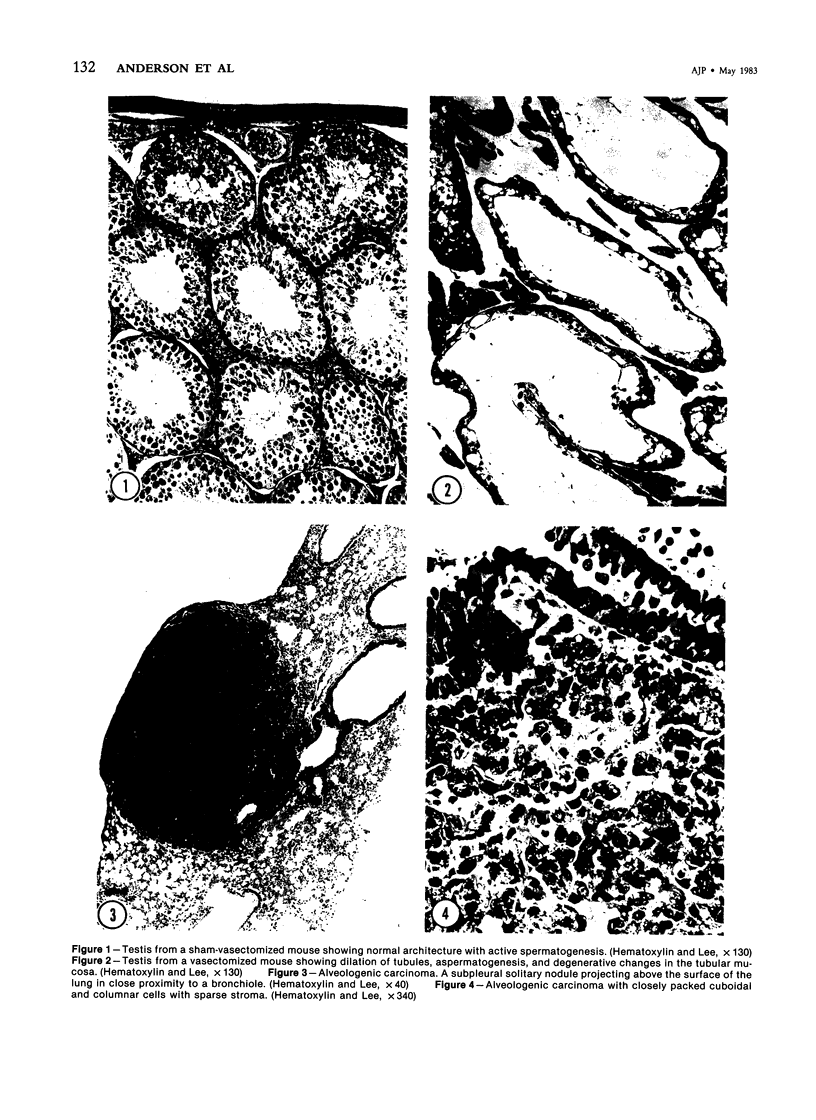
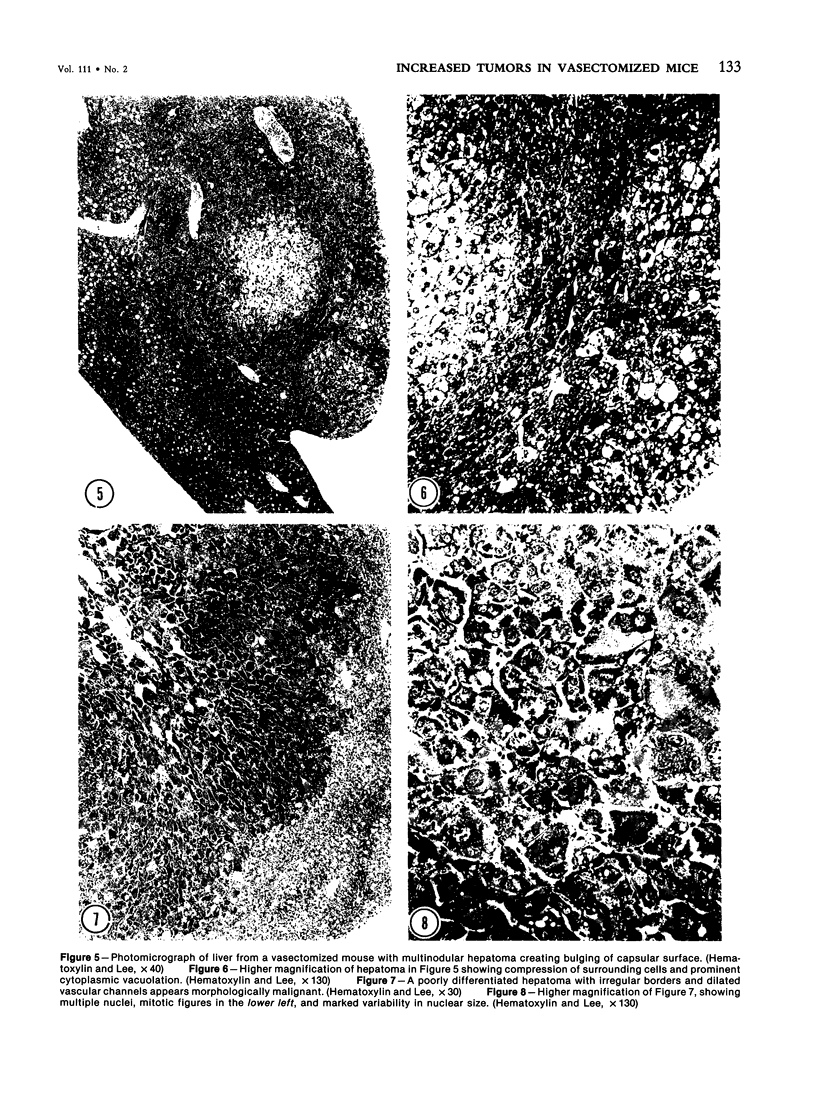


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander N. J., Anderson D. J. Vasectomy: consequences of autoimmunity to sperm antigens. Fertil Steril. 1979 Sep;32(3):253–260. doi: 10.1016/s0015-0282(16)44228-7. [DOI] [PubMed] [Google Scholar]
- Alexander N. J., Clarkson T. B. Vasectomy increases the severity of diet-induced atherosclerosis in Macaca fascicularis. Science. 1978 Aug 11;201(4355):538–541. doi: 10.1126/science.96532. [DOI] [PubMed] [Google Scholar]
- Anderson D. J., Alexander N. J. Antisperm antibody titres, immune complex deposition and immunocompetence in long-term vasectomized mice. Clin Exp Immunol. 1981 Jan;43(1):99–108. [PMC free article] [PubMed] [Google Scholar]
- Anderson D. J., Alexander N. J., Fulgham D. L., Vandenbark A. A., Burger D. R. Immunity to tumor-associated antigens in vasectomized men. J Natl Cancer Inst. 1982 Sep;69(3):551–555. [PubMed] [Google Scholar]
- Anderson D. J., Tarter T. H. Immunosuppressive effects of mouse seminal plasma components in vivo and in vitro. J Immunol. 1982 Feb;128(2):535–539. [PubMed] [Google Scholar]
- Bigazzi P. E., Kosuda L. L., Hsu K. C., Andres G. A. Immune complex orchitis in vasectomized rabbits. J Exp Med. 1976 Feb 1;143(2):382–404. doi: 10.1084/jem.143.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J. E., Hunt R., Lance E. M., Medawar P. B. Proceedings: Implications of the fetal antigen theory for fetal transplantation. Cancer Res. 1974 Aug;34(8):2055–2060. [PubMed] [Google Scholar]
- Chism S. E., Wallis S., Burton R. C., Warner N. L. Analysis of murine oncofetal antigens as tumor-associated transplantation antigens. J Immunol. 1976 Nov;117(5 PT2):1870–1877. [PubMed] [Google Scholar]
- Clarkson T. B., Alexander N. J. Long-term vasectomy: effects on the occurrence and extent of atherosclerosis in rhesus monkeys. J Clin Invest. 1980 Jan;65(1):15–25. doi: 10.1172/JCI109645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggin J. H., Jr, Anderson N. G. Cancer, differentiation and embryonic antigens: some central problems. Adv Cancer Res. 1974;19(0):105–165. doi: 10.1016/s0065-230x(08)60053-6. [DOI] [PubMed] [Google Scholar]
- Frith C. H., Wiley L. Spontaneous hepatocellular neoplasms and hepatic hemangiosarcomas in several strains of mice. Lab Anim Sci. 1982 Apr;32(2):157–162. [PubMed] [Google Scholar]
- Gachelin G., Kemler R., Kelly F., Jacob F. PCC4, a new cell surface antigen common to multipotential embryonal carcinoma cells, spermatozoa, and mouse early embryos. Dev Biol. 1977 May;57(1):199–209. doi: 10.1016/0012-1606(77)90365-7. [DOI] [PubMed] [Google Scholar]
- Goldberg E. H., Tokuda S. Evidence for related antigens on sperm, tumor, and fetal cells in the mouse. Transplant Proc. 1977 Jun;9(2):1363–1365. [PubMed] [Google Scholar]
- Hurtenbach U., Shearer G. M. Germ cell-induced immune suppression in mice. Effect of inoculation of syngeneic spermatozoa on cell-mediated immune responses. J Exp Med. 1982 Jun 1;155(6):1719–1729. doi: 10.1084/jem.155.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lausch R. N., Rapp F. Tumor-specific antigens and reexpression of fetal antigens in mammalian cells. Prog Exp Tumor Res. 1974;19:45–58. doi: 10.1159/000395848. [DOI] [PubMed] [Google Scholar]
- Lord E. M., Sensabaugh G. F., Stites D. P. Immunosuppressive activity of human seminal plasma. I. Inhibition of in vitro lymphocyte activation. J Immunol. 1977 May;118(5):1704–1711. [PubMed] [Google Scholar]
- Marcus Z. H., Freisheim J. H., Houk J. L., Herman J. H., Hess E. V. In vitro studies in reproductive immunology. 1. Suppression of cell-mediated immune response by human spermatozoa and fractions isolated from human seminal plasma. Clin Immunol Immunopathol. 1978 Mar;9(3):318–326. doi: 10.1016/0090-1229(78)90103-4. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Menge A. C., Fleming C. H. Detection of sperm antigens on mouse ova and early embryos. Dev Biol. 1978 Mar;63(1):111–117. doi: 10.1016/0012-1606(78)90117-3. [DOI] [PubMed] [Google Scholar]
- Muir V. Y., Turk J. L. Immunological unresponsiveness during induction of experimental autoimmune orchitis in guinea-pigs: studies in vivo and in vitro. Immunology. 1979 Jan;36(1):95–102. [PMC free article] [PubMed] [Google Scholar]
- Parmiani G., Lembo R. Effect of anti-embryo immunization on methylcholanthrene-induced sarcoma growth in BALB/c mice. Int J Cancer. 1974 Oct 15;14(4):555–564. doi: 10.1002/ijc.2910140416. [DOI] [PubMed] [Google Scholar]
- Solter D., Schachner M. Brain and sperm cell surface antigen (NS-4) on preimplantation mouse embryos. Dev Biol. 1976 Aug;52(1):98–104. doi: 10.1016/0012-1606(76)90010-5. [DOI] [PubMed] [Google Scholar]
- Teuscher C., Wild G. C., Johnson E., Tung K. S. Vasectomy (An experimental autoimmune disease state). Ric Clin Lab. 1981 Oct-Dec;11(4):313–329. [PubMed] [Google Scholar]
- Tung K. S., Alexander N. J. Monocytic orchitis and aspermatogenesis in normal and vasectomized rhesus macaques (Macaca mulatta). Am J Pathol. 1980 Oct;101(1):17–29. [PMC free article] [PubMed] [Google Scholar]
- Wilson B. J., Alexander N. J., Porter G., Fulgham D. L. Cell-mediated immunity in vasectomized rhesus monkeys. Fertil Steril. 1977 Dec;28(12):1349–1355. doi: 10.1016/s0015-0282(16)42983-3. [DOI] [PubMed] [Google Scholar]