Abstract
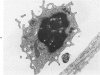
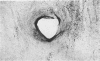
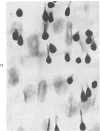
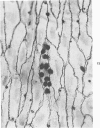
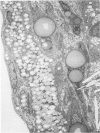
In rats with diet-induced hypercholesterolemia, two concomitant changes began to occur within 1 week and persisted for 1 year: an increase in total plasma cholesterol and an increase in the number of mononuclear cells adhering to the aortic intima (up to values 50 times normal). Adherent cells were approximately 90% monocytes and approximately 10% lymphocytes. Adhesion was focal, with some preference for ostia of aortic branches; it was followed by migration into the subendothelial space. The subendothelial monocytes/macrophages progressively became foam cells, thus giving rise to microscopic "fatty streaks." Ultimately, typical atherosclerotic plaques were formed. Four possible mechanisms of increased cell adhesion are suggested. Endothelial changes were mild; myelin figures arising from the endothelial surface were seen by electron microscopy. Endothelial denudation was never observed, neither in light-microscopic preparations stained with AgNO3 nor by ultrastructure. Platelet participation was minimal. It is concluded that in this model atherosclerotic plaques are initiated by mononuclear cell adhesion and emigration; endothelial denudation is not a necessary step in their pathogenesis.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRUS S. B., FILLIOS L. C., MANN G. V., STARE F. J. Experimental production of gross atherosclerosis in the rat. J Exp Med. 1956 Oct 1;104(4):539–554. doi: 10.1084/jem.104.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong M. L., Peterson R. E., Hoak J. C., Megan M. B., Cheng F. H., Clarke W. R. Arterial platelet accumulation in experimental hypercholesterolemia. Atherosclerosis. 1980 May;36(1):89–100. doi: 10.1016/0021-9150(80)90202-6. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Goldstein J. L. Mutations affecting the binding, internalization, and lysosomal hydrolysis of low density lipoprotein in cultured human fibroblasts, lymphocytes, and aortic smooth muscle cells. J Supramol Struct. 1977;6(1):85–94. doi: 10.1002/jss.400060107. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Basu S. K., Falck J. R., Ho Y. K., Goldstein J. L. The scavenger cell pathway for lipoprotein degradation: specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J Supramol Struct. 1980;13(1):67–81. doi: 10.1002/jss.400130107. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Ryan G. B., Breslow J. L., Karnovsky M. J. Absence of enhanced intimal thickening in the response of the carotid arterial wall to endothelial injury in hypercholesterolemic rats. Lab Invest. 1976 Jul;35(1):6–17. [PubMed] [Google Scholar]
- Dvorak H. F., Dvorak A. M. Basophilic leucocytes: structure, function and role in disease. Clin Haematol. 1975 Oct;4(3):651–683. [PubMed] [Google Scholar]
- ESTERLY J. A., GLAGOV S. ALTERED PERMEABILITY OF THE RENAL ARTERY OF THE HYPERTENSIVE RAT: AN ELECTRON MICROSCOPIC STUDY. Am J Pathol. 1963 Oct;43:619–638. [PMC free article] [PubMed] [Google Scholar]
- Florentin R. A., Nam S. C., Lee K. T., Lee K. J., Thomas W. A. Increased mitotic activity in aortas of swine after three days of cholesterol feeding. Arch Pathol. 1969 Nov;88(5):463–469. [PubMed] [Google Scholar]
- Fritz K. E., Daoud A. S., Jarmolych J. Study of esterase-positive cells in swine atherosclerosis. Artery. 1980;8(3):220–224. [PubMed] [Google Scholar]
- GRESHAM G. A., HOWARD A. N. The independent production of atherosclerosis and thrombosis in the rat. Br J Exp Pathol. 1960 Aug;41:395–402. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K. Lipid clearance from fatty streak lesions by foam cell migration. Artery. 1980;8(3):215–219. [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K., Richardson M., Schwartz C. J. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol. 1979 Jun;95(3):775–792. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K. Ultrastructural identification of monocyte-derived foam cells in fatty streak lesions. Artery. 1980;8(3):208–214. [PubMed] [Google Scholar]
- Gerrity R. G., Schwartz C. J. Endothelial cell injury in early mild hypercholesterolemia. Prog Biochem Pharmacol. 1977;13:213–219. [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981 May;103(2):191–200. [PMC free article] [PubMed] [Google Scholar]
- Henriksen T., Evensen S. A., Carlander B. Injury to human endothelial cells in culture induced by low density lipoproteins. Scand J Clin Lab Invest. 1979 Jun;39(4):361–368. doi: 10.3109/00365517909106120. [DOI] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Interactions of plasma lipoproteins with endothelial cells. Ann N Y Acad Sci. 1982;401:102–116. doi: 10.1111/j.1749-6632.1982.tb25711.x. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Folger R., Haering W. A., Ware B. R., Karnovsky M. J. Adhesion of leukocytes to endothelium: roles of divalent cations, surface charge, chemotactic agents and substrate. J Cell Sci. 1980 Oct;45:73–86. doi: 10.1242/jcs.45.1.73. [DOI] [PubMed] [Google Scholar]
- Ingerman-Wojenski C. M., Sedar A. W., Nissenbaum M., Silver M. J., Klurfeld D. M., Kritchevsky D. Early morphological changes in the endothelium of a peripheral artery of rabbits fed an atherogenic diet. Exp Mol Pathol. 1983 Feb;38(1):48–60. doi: 10.1016/0014-4800(83)90097-7. [DOI] [PubMed] [Google Scholar]
- Jauchem J. R., Lopez M., Sprague E. A., Schwartz C. J. Mononuclear cell chemoattractant activity from cultured arterial smooth muscle cells. Exp Mol Pathol. 1982 Oct;37(2):166–174. doi: 10.1016/0014-4800(82)90033-8. [DOI] [PubMed] [Google Scholar]
- Jorgensen L., Packham M. A., Rowsell H. C., Mustard J. F. Deposition of formed elements of blood on the intima and signs of intimal injury in the aorta of rabbit, pig, and man. Lab Invest. 1972 Sep;27(3):341–350. [PubMed] [Google Scholar]
- Joris I., Stetz E., Majno G. Lymphocytes and monocytes in the aortic intima--An electron-microscopic study in the rat. Atherosclerosis. 1979 Nov;34(3):221–231. doi: 10.1016/s0021-9150(79)80003-9. [DOI] [PubMed] [Google Scholar]
- Joris I., Zand T., Majno G. Hydrodynamic injury of the endothelium in acute aortic stenosis. Am J Pathol. 1982 Mar;106(3):394–408. [PMC free article] [PubMed] [Google Scholar]
- Kurozumi T. Electron microscopic study on permeability of the aorta and basilar artery of the rabbit--with special reference to the changes of permeability by hypercholesteremia. Exp Mol Pathol. 1975 Aug;23(1):1–11. doi: 10.1016/0014-4800(75)90002-7. [DOI] [PubMed] [Google Scholar]
- Lewis J. C., Taylor R. G., Jones N. D., St Clair R. W., Cornhill J. F. Endothelial surface characteristics in pigeon coronary artery atherosclerosis. I. Cellular alterations during the initial stages of dietary cholesterol challenge. Lab Invest. 1982 Feb;46(2):123–138. [PubMed] [Google Scholar]
- Massmann J. Mononuclear cell infiltration of the aortic intima in domestic swine. Exp Pathol (Jena) 1979;17(2):110–112. doi: 10.1016/s0014-4908(79)80035-6. [DOI] [PubMed] [Google Scholar]
- Packham M. A., Rowsell H. C., Jorgensen L., Mustard J. F. Localized protein accumulation in the wall of the aorta. Exp Mol Pathol. 1967 Oct;7(2):214–232. doi: 10.1016/0014-4800(67)90031-7. [DOI] [PubMed] [Google Scholar]
- Potvliege P. R., Bourgain R. H. The effect of a fat-rich diet on the ultrastructure of mesenteric arteries of the rat and their reaction to local desendothelialization. Br J Exp Pathol. 1982 Feb;63(1):116–123. [PMC free article] [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial injury and regeneration. IV. Endotoxin: a nondenuding injury to aortic endothelium. Lab Invest. 1983 Jan;48(1):25–34. [PubMed] [Google Scholar]
- Ross R. George Lyman Duff Memorial Lecture. Atherosclerosis: a problem of the biology of arterial wall cells and their interactions with blood components. Arteriosclerosis. 1981 Sep-Oct;1(5):293–311. doi: 10.1161/01.atv.1.5.293. [DOI] [PubMed] [Google Scholar]
- Ross R., Harker L. Hyperlipidemia and atherosclerosis. Science. 1976 Sep 17;193(4258):1094–1100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- STILL W. J., MARRIOTT P. R. COMPARATIVE MORPHOLOGY OF THE EARLY ATHEROSCLEROTIC LESION IN MAN AND CHOLESTEROL-ATHEROSCLEROSIS IN THE RABBIT AN ELECTRONMICROSCOPIC STUDY. J Atheroscler Res. 1964 Sep-Oct;4:373–386. doi: 10.1016/s0368-1319(64)80023-5. [DOI] [PubMed] [Google Scholar]
- STILL W. J., O'NEAL R. M. Electron microscopic study of experimental atherosclerosis in the rat. Am J Pathol. 1962 Jan;40:21–35. [PMC free article] [PubMed] [Google Scholar]
- STILL W. J. PATHOGENESIS OF EXPERIMENTAL ATHEROSCLEROSIS. Arch Pathol. 1964 Dec;78:601–612. [PubMed] [Google Scholar]
- Schaper J., König R., Franz D., Schaper W. The endothelial surface of growing coronary collateral arteries. Intimal margination and diapedesis of monocytes. A combined SEM and TEM study. Virchows Arch A Pathol Anat Histol. 1976 Jun 22;370(3):193–205. doi: 10.1007/BF00427580. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M. Role of endothelial integrity in atherosclerosis. Artery. 1980;8(4):305–314. [PubMed] [Google Scholar]
- Silkworth J. B., McLean B., Stehbens W. E. The effect of hypercholesterolemia on aortic endothelium studied en face. Atherosclerosis. 1975 Nov-Dec;22(3):335–348. doi: 10.1016/0021-9150(75)90015-5. [DOI] [PubMed] [Google Scholar]
- Stary H. C. Coronary artery fine structure in rhesus monkeys: the early atherosclerotic lesion and its progression. Primates Med. 1976;9:359–395. [PubMed] [Google Scholar]
- Stary H. C. The intimal macrophage in atherosclerosis. Artery. 1980;8(3):205–207. [PubMed] [Google Scholar]
- Stemerman M. B. Effects of moderate hypercholesterolemia on rabbit endothelium. Arteriosclerosis. 1981 Jan-Feb;1(1):25–32. doi: 10.1161/01.atv.1.1.25. [DOI] [PubMed] [Google Scholar]
- Still W. J., Dennison S. The arterial endothelium of the hypertensive rat: a scanning and transmission electron microscopical study. Arch Pathol. 1974 Jun;97(6):337–342. [PubMed] [Google Scholar]
- Still W. J. Hyperlipemia and the arterial intima of the hypertensive rat. Arch Pathol. 1970 May;89(5):392–404. [PubMed] [Google Scholar]
- Still W. J. The pathogenesis of the intimal thickenings produced by hypertension in large arteries in the rat. Lab Invest. 1968 Jul;19(1):84–91. [PubMed] [Google Scholar]
- Tauber J. P., Goldminz D., Vlodavsky I., Gospodarowicz D. The interaction of the high-density lipoprotein with cultured cells of bovine vascular endothelium. Eur J Biochem. 1981 Oct;119(2):317–325. doi: 10.1111/j.1432-1033.1981.tb05611.x. [DOI] [PubMed] [Google Scholar]
- Thomas W. A., Kim D. N. Biology of disease. Atherosclerosis as a hyperplastic and/or neoplastic process. Lab Invest. 1983 Mar;48(3):245–255. [PubMed] [Google Scholar]
- Todd M. E., Friedman S. M. The ultrastructure of peripheral arteries during the development of DOCA hypertension in the rat. Z Zellforsch Mikrosk Anat. 1972;128(4):538–554. doi: 10.1007/BF00306987. [DOI] [PubMed] [Google Scholar]
- Traber M. G., Kayden H. J. Low density lipoprotein receptor activity in human monocyte-derived macrophages and its relation to atheromatous lesions. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5466–5470. doi: 10.1073/pnas.77.9.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trillo A. A. The cell population of aortic fatty streaks in African green monkeys with special reference to granulocytic cells. An ultrastructural study. Atherosclerosis. 1982 Jun;43(2-3):259–275. doi: 10.1016/0021-9150(82)90027-2. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Fielding P. E., Johnson L. K., Gospodarowicz D. Inhibition of low density lipoprotein uptake in confluent endothelial cell monolayers correlates with a restricted surface receptor redistribution. J Cell Physiol. 1979 Sep;100(3):481–495. doi: 10.1002/jcp.1041000311. [DOI] [PubMed] [Google Scholar]
- WISSLER R. W., EILERT M. L., SCHROEDER M. A., COHEN L. Production of lipomatous and atheromatous arterial lesions in the albino rat. AMA Arch Pathol. 1954 Apr;57(4):333–351. [PubMed] [Google Scholar]
- Wright H. P. Endothelial mitosis around aortic branches in normal guinea pigs. Nature. 1968 Oct 5;220(5162):78–79. doi: 10.1038/220078a0. [DOI] [PubMed] [Google Scholar]
- Yi P. I., Beck G., Zucker S. Rat plasma lipoprotein inhibitors of lymphocyte proliferation: specific membrane receptor for very low density lipoproteins. Int Arch Allergy Appl Immunol. 1981;65(1):8–14. doi: 10.1159/000232731. [DOI] [PubMed] [Google Scholar]
- de Bono D. Endothelial-lymphocyte interactions in vitro. I. Adherence of nonallergised lymphocytes. Cell Immunol. 1976 Sep;26(1):78–88. doi: 10.1016/0008-8749(76)90349-x. [DOI] [PubMed] [Google Scholar]
- de Bono D. Endothelium-lymphocyte interactions in vitro. II. Adherence of allergized lymphocytes. Cell Immunol. 1979 Apr;44(1):64–70. doi: 10.1016/0008-8749(79)90028-5. [DOI] [PubMed] [Google Scholar]