Abstract
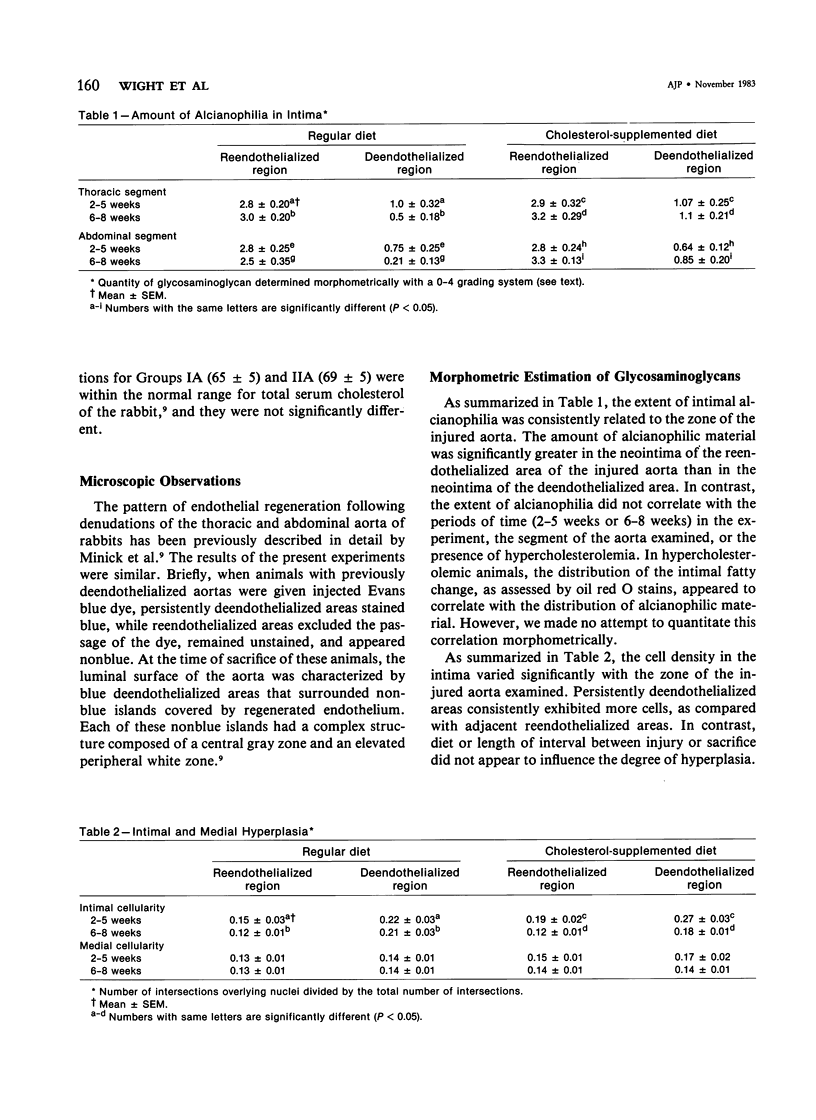
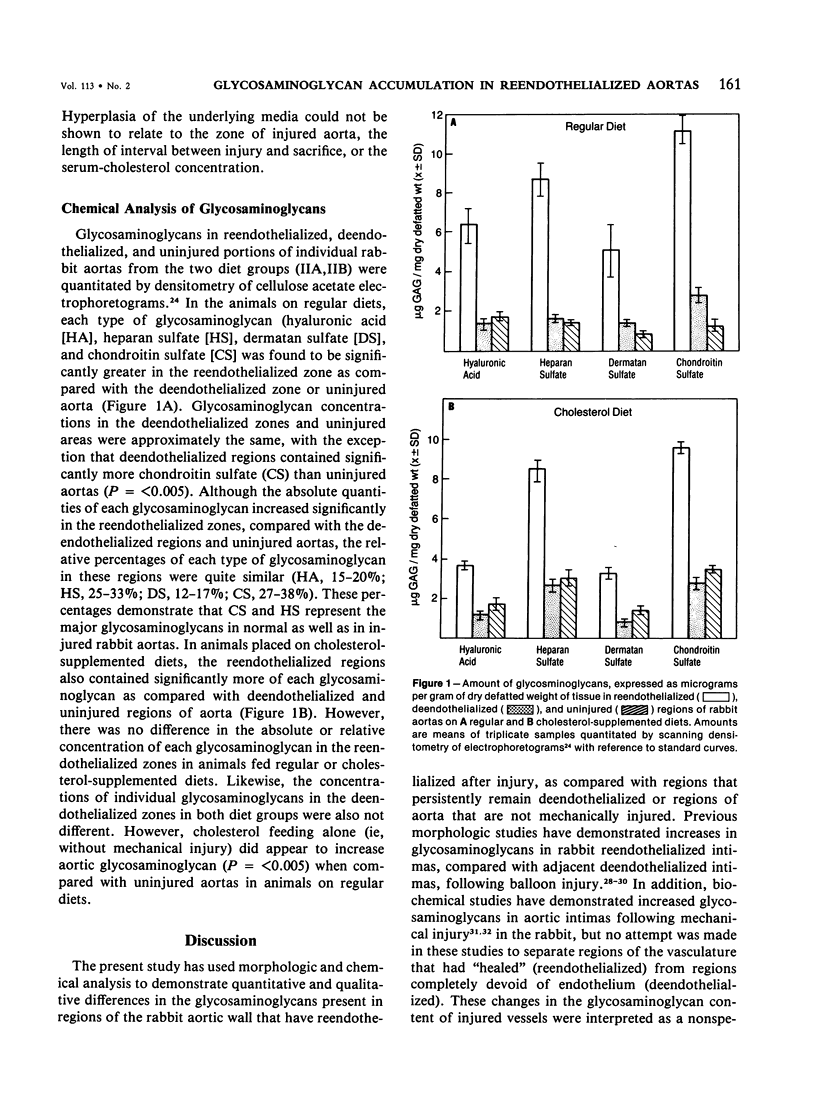
Previous studies have indicated that reendothelialized regions of injured rabbit aortas are more susceptible to diet-induced atherosclerosis than persistently deendothelialized regions or uninjured aortas. However, the mechanism responsible for this selective lipid deposition is not understood. One possibility is that these regions differ with respect to the quantity and type of glycosaminoglycan-containing proteoglycans which are known to interact with lipoproteins. To determine whether these regions differed with respect to their glycosaminoglycan composition, the authors divided 53 rabbits into four groups. Groups IA and IB were fed a regular diet beginning 5 weeks prior to aortic deendothelialization; Groups IIA and IIB were fed the same diet supplemented with 0.5% cholesterol. The rabbits were continued on these diets following aortic deendothelialization with a balloon catheter. Those in Groups IA and IIA were sacrificed either at 2-5 weeks or 6-8 weeks following deendothelialization; proteoglycans were assessed morphometrically following staining with alcian blue. Groups IB and IIB were sacrificed at 10 weeks following injury; glycosaminoglycans were extracted from deendothelialized and reendothelialized aortas, separated by electrophoresis, and quantitated by scanning densitometry. Morphometric analysis of stained aortic sections revealed significantly increased quantities of alcianophilic material in the neointima of reendothelialized aortas as compared with deendothelialized aortas in both diet groups. Chemical analysis revealed significantly more of each glycosaminoglycan in reendothelialized aortas when compared with deendothelialized or uninjured aortas. The major glycosaminoglycans present in all regions were heparan sulfate and chondroitin sulfate; and although absolute quantities of these particular glycosaminoglycans increased in the reendothelialized region, their relative percentages remained the same for each area analyzed. Cholesterol feeding did not appear to influence glycosaminoglycan concentration and composition in reendothelialized and deendothelialized regions when compared with normal diets, but cholesterol feeding alone did increase aortic glycosaminoglycans in uninjured aortas. The results suggest that the presence of endothelium influences the quantity and type of glycosaminoglycans accumulating in the neointima, and that the differences in proteoglycans in the reendothelialized artery may account at least in part for the propensity of this area to accumulate lipid and evolve as atherosclerosis.
Full text
PDF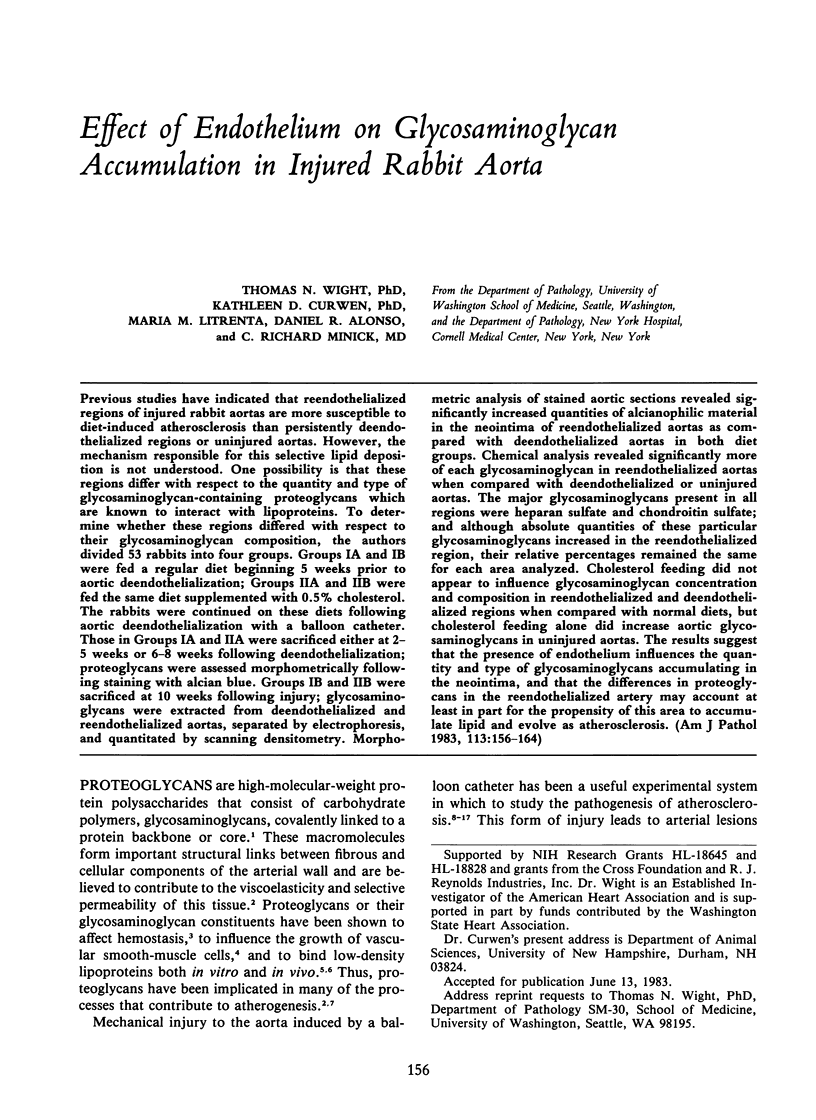






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anseth A. Influence of corneal epithelium on the incorporation of 35SO4 into stromal glycosaminoglycans. Exp Eye Res. 1971 Mar;11(2):251–254. doi: 10.1016/s0014-4835(71)80029-5. [DOI] [PubMed] [Google Scholar]
- BUCK R. C., HEAGY F. C. Uptake of radioactive sulphur by various tissues of normal and cholesterol-fed rabbits. Can J Biochem Physiol. 1958 Jan;36(1):63–69. [PubMed] [Google Scholar]
- BUCK R. C. Uptake of radioactive sulfate by arteries of normal and cholesterol-fed rabbits. J Histochem Cytochem. 1955 Nov;3(6):435–440. doi: 10.1177/3.6.435. [DOI] [PubMed] [Google Scholar]
- Baumgartner H. R., Stemerman M. B., Spaet T. H. Adhesion of blood platelets to subendothelial surface: distinct from adhesion to collagen. Experientia. 1971 Mar 15;27(3):283–285. doi: 10.1007/BF02138148. [DOI] [PubMed] [Google Scholar]
- Berenson G. S., Radhakrishnamurthy B., Dalferes E. R., Jr, Srinivasan S. R. Carbohydrate macromolecules and atherosclerosis. Hum Pathol. 1971 Mar;2(1):57–79. doi: 10.1016/s0046-8177(71)80021-7. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Buonassisi V. Sulfated mucopolysaccharide synthesis and secretion in endothelial cell cultures. Exp Cell Res. 1973 Feb;76(2):363–368. doi: 10.1016/0014-4827(73)90388-1. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B. C., Chemnitz J., Tkocz I., Kim C. M. Repair in arterial tissue. 2. Connective tissue changes following an embolectomy catheter lesion. The importance of the endothelial cells to repair and regeneration. Acta Pathol Microbiol Scand A. 1979 Jul;87A(4):275–283. [PubMed] [Google Scholar]
- Cremer-Bartels G., Grundmann E., Mielonen P., Buddecke E. Light-induced variation of glucose metabolism in bovine cornea. Exp Eye Res. 1972 Mar;13(2):172–177. doi: 10.1016/0014-4835(72)90030-9. [DOI] [PubMed] [Google Scholar]
- Curwen K. D., Smith S. C. Quantitative microanalysis of aortic glycosaminoglycans. Anal Biochem. 1977 May 1;79(1-2):291–301. doi: 10.1016/0003-2697(77)90404-3. [DOI] [PubMed] [Google Scholar]
- Derby M. A., Pintar J. E. The histochemical specificity of Streptomyces hyaluronidase and chondroitinase ABC. Histochem J. 1978 Sep;10(5):529–547. doi: 10.1007/BF01003135. [DOI] [PubMed] [Google Scholar]
- Ehrlich K., Murray M. Effect of low density lipoprotein on proteoglycan synthesis by aorta cells in culture. Experientia. 1978 Feb 15;34(2):179–181. doi: 10.1007/BF01944662. [DOI] [PubMed] [Google Scholar]
- Falcone D. J., Hajjar D. P., Minick C. R. Enhancement of cholesterol and cholesteryl ester accumulation in re-endothelialized aorta. Am J Pathol. 1980 Apr;99(1):81–104. [PMC free article] [PubMed] [Google Scholar]
- Gamse G., Fromme H. G., Kresse H. Metabolism of sulfated glycosaminoglycans in cultured endothelial cells and smooth muscle cells from bovine aorta. Biochim Biophys Acta. 1978 Dec 18;544(3):514–528. doi: 10.1016/0304-4165(78)90326-4. [DOI] [PubMed] [Google Scholar]
- Hajjar D. P., Falcone D. J., Fowler S., Minick C. R. Endothelium modifies the altered metabolism of the injured aortic wall. Am J Pathol. 1981 Jan;102(1):28–39. [PMC free article] [PubMed] [Google Scholar]
- Helin G., Helin P., Lorenzen I. The aortic glycosaminoglycans in arteriosclerosis induced by systemic hypoxia. Atherosclerosis. 1970 Sep-Oct;12(2):235–240. doi: 10.1016/0021-9150(70)90102-4. [DOI] [PubMed] [Google Scholar]
- Helin P., Garbarsch C., Lorenzen I. Effects of intermittent and continuous hypoxia on the aortic wall in rabbits. Atherosclerosis. 1975 May-Jun;21(3):325–335. doi: 10.1016/0021-9150(75)90046-5. [DOI] [PubMed] [Google Scholar]
- Helin P., Lorenzen I., Garbarsch C., Matthiessen M. E. Repair in arterial tissue. Morphological and biochemical changes in rabbit aorta after a single dilatation injury. Circ Res. 1971 Nov;29(5):542–554. doi: 10.1161/01.res.29.5.542. [DOI] [PubMed] [Google Scholar]
- Ho K. J., Forestner J. E., Manalo-Estrella P. Aortic acid mucopolysaccharides: changes during pregnancy, enovid-treatment, and hypercholesteremia in rabbits. Proc Soc Exp Biol Med. 1971 May;137(1):10–12. doi: 10.3181/00379727-137-35501. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Rosenberg R., Haering W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. II. In vitro studies. Circ Res. 1980 Oct;47(4):578–583. doi: 10.1161/01.res.47.4.578. [DOI] [PubMed] [Google Scholar]
- Ichida T., Kalant N. Aortic glycosaminoglycans in atheroma and alloxan diabetes. Can J Biochem. 1968 Mar;46(3):249–260. doi: 10.1139/o68-036. [DOI] [PubMed] [Google Scholar]
- Iverius P. H. The interaction between human plasma lipoproteins and connective tissue glycosaminoglycans. J Biol Chem. 1972 Apr 25;247(8):2607–2613. [PubMed] [Google Scholar]
- Klintworth G. K., Smith C. F. A comparative study of extracellular sulfated glycosaminoglycans synthesized by rabbit corneal fibroblasts in organ and confluent cultures. Lab Invest. 1976 Sep;35(3):258–263. [PubMed] [Google Scholar]
- Kramer R. H., Vogel K. G., Nicolson G. L. Solubilization and degradation of subendothelial matrix glycoproteins and proteoglycans by metastatic tumor cells. J Biol Chem. 1982 Mar 10;257(5):2678–2686. [PubMed] [Google Scholar]
- Kresse H., von Figura K., Buddecke E., Fromme H. G. Metabolism of sulfated glycosaminoglycans in cultivated bovine arterial cells. I. Characterization of different pools of sulfated glycosaminoglycans. Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):929–941. doi: 10.1515/bchm2.1975.356.s1.929. [DOI] [PubMed] [Google Scholar]
- LEVINE J. B., ZAK B. AUTOMATED DETERMINATION OF SERUM TOTAL CHOLESTEROL. Clin Chim Acta. 1964 Oct;10:381–384. doi: 10.1016/0009-8981(64)90073-7. [DOI] [PubMed] [Google Scholar]
- LINKER A., MEYER K., HOFFMAN P. The production of hyaluronate oligosaccharides by leech hyaluronidase and alkali. J Biol Chem. 1960 Apr;235:924–927. [PubMed] [Google Scholar]
- Leung D. Y., Glagov S., Mathews M. B. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science. 1976 Feb 6;191(4226):475–477. doi: 10.1126/science.128820. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Lippiello L. The glycosaminoglycans of normal and arthritic cartilage. J Clin Invest. 1971 Aug;50(8):1712–1719. doi: 10.1172/JCI106660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawhinney T. P., Augustyn J. M., Fritz K. E. Glycosaminoglycan-lipoprotein complexes from aortas of hypercholesterolemic rabbits. Part 1. Isolation and characterization. Atherosclerosis. 1978 Oct;31(2):155–167. doi: 10.1016/0021-9150(78)90161-2. [DOI] [PubMed] [Google Scholar]
- Merrilees M. J., Merrilees M. A., Birnbaum P. S., Scott P. J., Flint M. H. The effect of centrifugal force on glycosaminoglycan production by aortic smooth muscle cells in culture. Atherosclerosis. 1977 Jul;27(3):259–264. doi: 10.1016/0021-9150(77)90034-x. [DOI] [PubMed] [Google Scholar]
- Merrilees M. J., Scott L. Interaction of aortic endothelial and smooth muscle cells in culture. Effect on glycosaminoglycan levels. Atherosclerosis. 1981 May;39(2):147–161. doi: 10.1016/0021-9150(81)90064-2. [DOI] [PubMed] [Google Scholar]
- Minick C. R., Litrenta M. M., Alonso D. R., Silane M. F., Stemerman M. B. Further studies on the effect of regenerated endothelium on intimal lipid accumulation. Prog Biochem Pharmacol. 1977;13:115–122. [PubMed] [Google Scholar]
- Minick C. R., Stemerman M. B., Insull W., Jr Role of endothelium and hypercholesterolemia in intimal thickening and lipid accumulation. Am J Pathol. 1979 Apr;95(1):131–158. [PMC free article] [PubMed] [Google Scholar]
- Minick C. R., Stemerman M. G., Insull W., Jr Effect of regenerated endothelium on lipid accumulation in the arterial wall. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1724–1728. doi: 10.1073/pnas.74.4.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Belbeck L. W., Richardson M., Taylor W. Lipid accumulation in the neointima formed in normally fed rabbits in response to one or six removals of the aortic endothelium. Lab Invest. 1982 Jul;47(1):37–42. [PubMed] [Google Scholar]
- Namiki O., Faris B., Tschopp F., Füglistaller P., Hollander W., Franzblau C., Schmid K. Synthesis of glycosaminoglycans by cultured rabbit smooth muscle cells. Biochemistry. 1980 Apr 29;19(9):1900–1904. doi: 10.1021/bi00550a026. [DOI] [PubMed] [Google Scholar]
- Palatý V., Gustafson B. K., Friedman S. M. The role of protein-polysaccharides in hydration of the arterial wall. Can J Physiol Pharmacol. 1970 Jan;48(1):54–60. doi: 10.1139/y70-009. [DOI] [PubMed] [Google Scholar]
- Radhakrishnamurthy B., Ruiz H. A., Jr, Berenson G. S. Isolation and characterization of proteoglycans from bovine aorta. J Biol Chem. 1977 Jul 25;252(14):4831–4841. [PubMed] [Google Scholar]
- Richardson M., Ihnatowycz I., Moore S. Glycosaminoglycan distribution in rabbit aortic wall following balloon catheter deendothelialization. An ultrastructural study. Lab Invest. 1980 Dec;43(6):509–516. [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Saxena I. D., Nagchaudhuri J. A study of the acid mucopolysaccharides of the rabbit aorta during cholesterol-induced atherosclerosis. Indian J Med Res. 1969 Jan;57(1):96–102. [PubMed] [Google Scholar]
- Saxena I. D., Nagchaudhuri J. Effect of high fat diets on acid mucopolysaccharides of the aorta in rabbits. Indian J Exp Biol. 1970 Jan;8(1):15–18. [PubMed] [Google Scholar]
- Smith E. B., Staples E. M., Dietz H. S., Smith R. H. Role of endothelium in sequestration of lipoprotein and firbrinogen in aortic lesions, thrombi, and graft pseudo-intimas. Lancet. 1979 Oct 20;2(8147):812–816. doi: 10.1016/s0140-6736(79)92173-1. [DOI] [PubMed] [Google Scholar]
- Spaet T. H., Rhee C., Geiger C. Delayed consequences of endothelial removal from rabbit aortae. Adv Exp Med Biol. 1978;102:165–173. doi: 10.1007/978-1-4757-1217-9_10. [DOI] [PubMed] [Google Scholar]
- Srinivasan S. R., Radhakrishnamurthy B., Pargaonkar P. S., Berenson G. S., Dolan P. Lipoprotein-acid mucopolysaccharide complexes of human atherosclerotic lesions. Biochim Biophys Acta. 1975 Apr 18;388(1):58–70. doi: 10.1016/0005-2760(75)90062-4. [DOI] [PubMed] [Google Scholar]
- Vijayagopal P., Radhakrishnamurthy B., Srinivasan S. R., Berenson G. S. Studies of biologic properties of proteoglycans from bovine aorta. Lab Invest. 1980 Feb;42(2):190–196. [PubMed] [Google Scholar]
- Wight T. N. Differences in the synthesis and secretion of sulfated glycosaminoglycans by aorta explant monolayers cultured from atherosclerosis-susceptible and -resistant pigeons. Am J Pathol. 1980 Oct;101(1):127–142. [PMC free article] [PubMed] [Google Scholar]
- Wight T. N., Hascall V. C. Proteoglycans in primate arteries. III. Characterization of the proteoglycans synthesized by arterial smooth muscle cells in culture. J Cell Biol. 1983 Jan;96(1):167–176. doi: 10.1083/jcb.96.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight T. N., Ross R. Proteoglycans in primate arteries. II. Synthesis and secretion of glycosaminoglycans by arterial smooth muscle cells in culture. J Cell Biol. 1975 Dec;67(3):675–686. doi: 10.1083/jcb.67.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Berlepsch K., Studer A. Acid mucopolysaccharides after overdilatation of the aortic wall of the rabbit. Angiologica. 1969;6(2):105–110. doi: 10.1159/000157783. [DOI] [PubMed] [Google Scholar]



