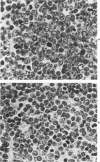
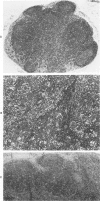
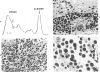
Abstract
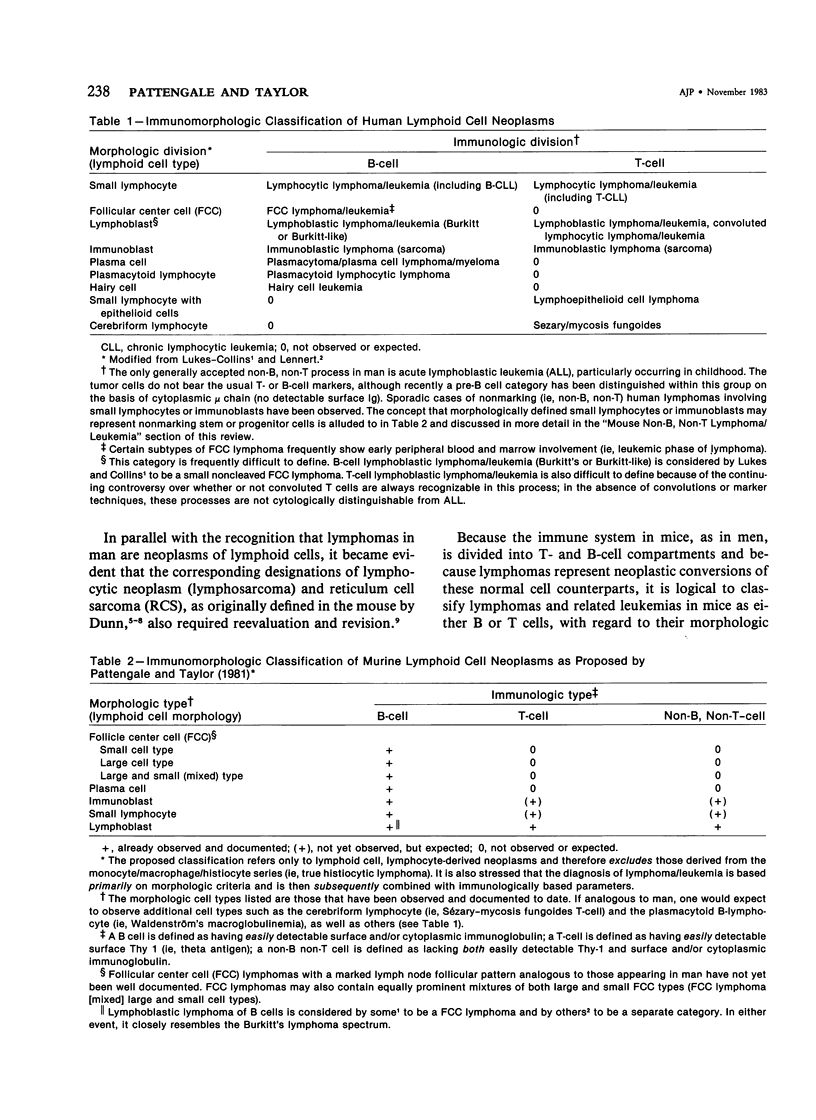
The present review focuses on the mouse as an experimental immunopathologic model for human non-Hodgkin's lymphomas and related leukemias. Immunomorphologic evidence is presented that clearly demonstrates that B- and T-cell subtypes of mouse (murine) lymphoma/leukemia closely resemble and are analogous to B- and T-cell subtypes of human lymphoma/leukemia as defined by recently proposed immunomorphologic classifications. Further evidence is presented that favors the hypothesis that certain types of murine and human B-cell lymphoma develop out of prodromal, prelymphomatous states, which exhibit antecedent morphologic and immunologic abnormalities. The many experimental advantages of the murine systems are stressed, as well as the concept that the presently defined immunomorphologic approach should be effectively combined with molecular and cytogenetic parameters.
Full text
PDF















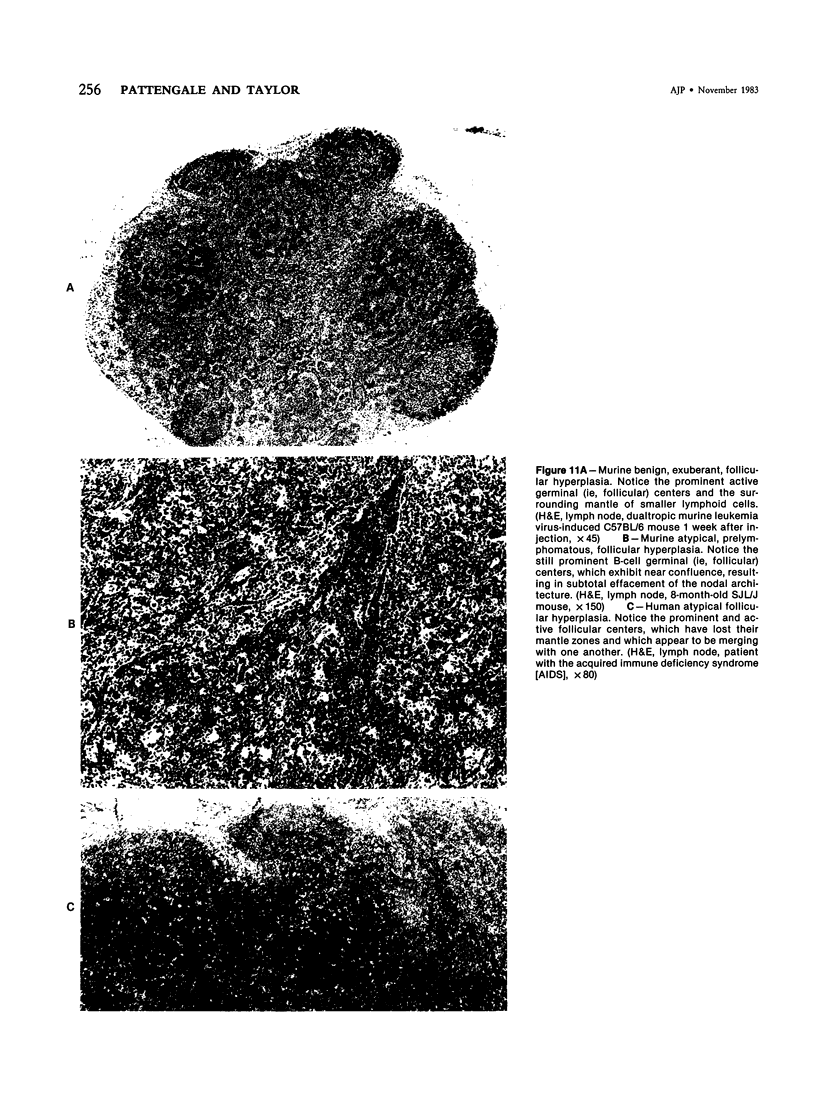
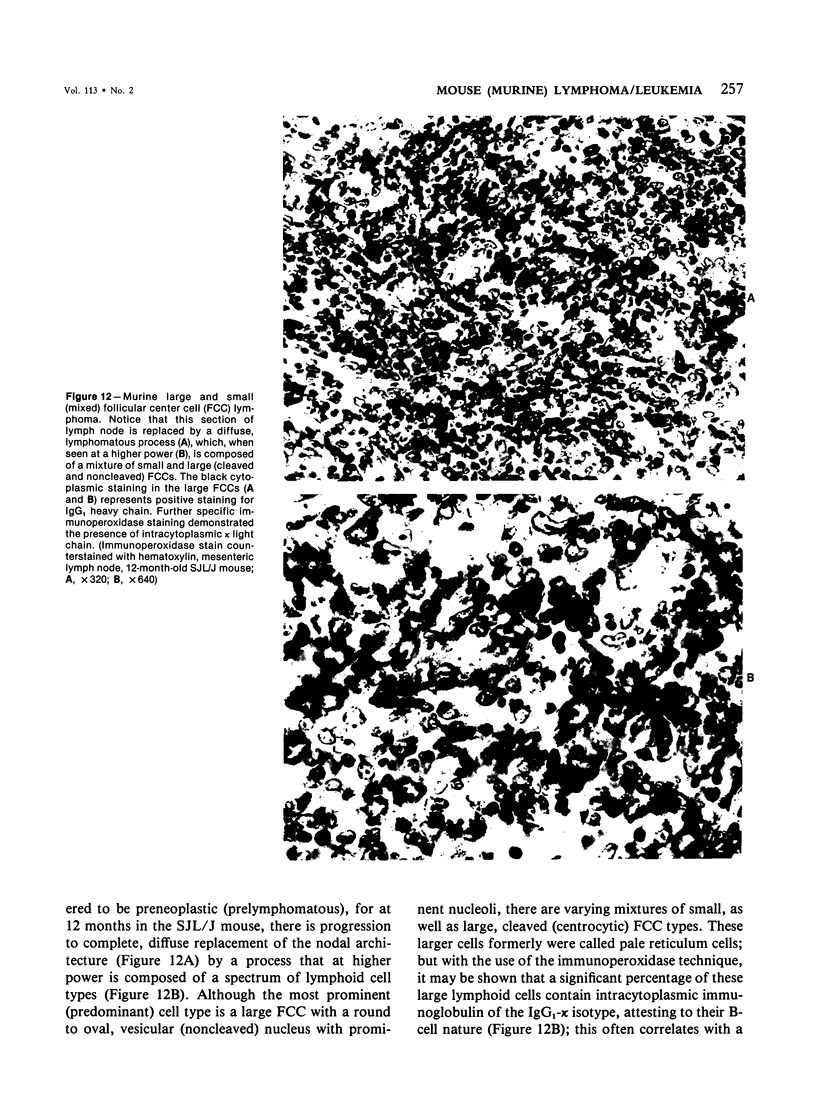


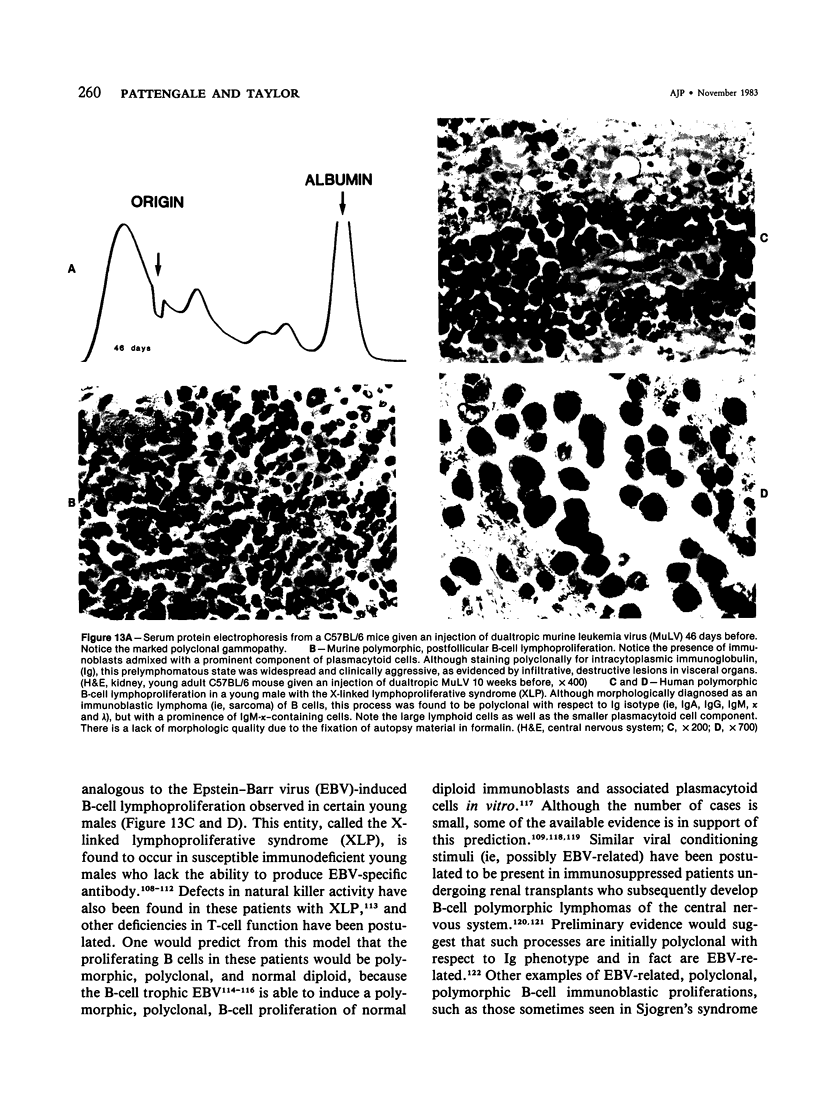





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Buxbaum J., Citronbaum R., Douglas S., Forni L., Melchers F., Pernis B., Stott D. IgM-producing tumors in the BALB-c mouse: a model for B-cell maturation. J Exp Med. 1974 Sep 1;140(3):742–763. doi: 10.1084/jem.140.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong M. Y., Gleichmann E., Gleichmann H., Beldotti L., Andre-Schwartz J., Schwartz R. S. Chronic allogeneic disease. II. Development of lymphomas. J Exp Med. 1970 Sep 1;132(3):417–439. doi: 10.1084/jem.132.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes R. D., Wills E. J. "Normal" elimination of aberrant autoimmune clones. Lancet. 1976 Jul 3;2(7975):20–21. doi: 10.1016/s0140-6736(76)92970-6. [DOI] [PubMed] [Google Scholar]
- Barthold D. R., Kysela S., Steinberg A. D. Decline in suppressor T cell function with age in female NZB mice. J Immunol. 1974 Jan;112(1):9–16. [PubMed] [Google Scholar]
- Ben-Yaakov M., Haran-Ghera N. T & B lymphocytes in thymus of SJL/J mice. Nature. 1975 May 1;255(5503):64–66. doi: 10.1038/255064a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Oncogenes. Sci Am. 1982 Mar;246(3):80–92. doi: 10.1038/scientificamerican0382-80. [DOI] [PubMed] [Google Scholar]
- Botzenhardt U., Klein J., Ziff M. Primary in vitro cell-mediated lympholysis reaction of NZB mice against unmodified targets syngeneic at the major histocompatibility complex. J Exp Med. 1978 May 1;147(5):1435–1448. doi: 10.1084/jem.147.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein N. A., Allison A. C. Effect of antilymphocytic serum on the appearance of reticular neoplasms in SJL-J mice. Nature. 1970 Mar 21;225(5238):1139–1140. doi: 10.1038/2251139a0. [DOI] [PubMed] [Google Scholar]
- Calame K., Kim S., Lalley P., Hill R., Davis M., Hood L. Molecular cloning of translocations involving chromosome 15 and the immunoglobulin C alpha gene from chromosome 12 in two murine plasmacytomas. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6994–6998. doi: 10.1073/pnas.79.22.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
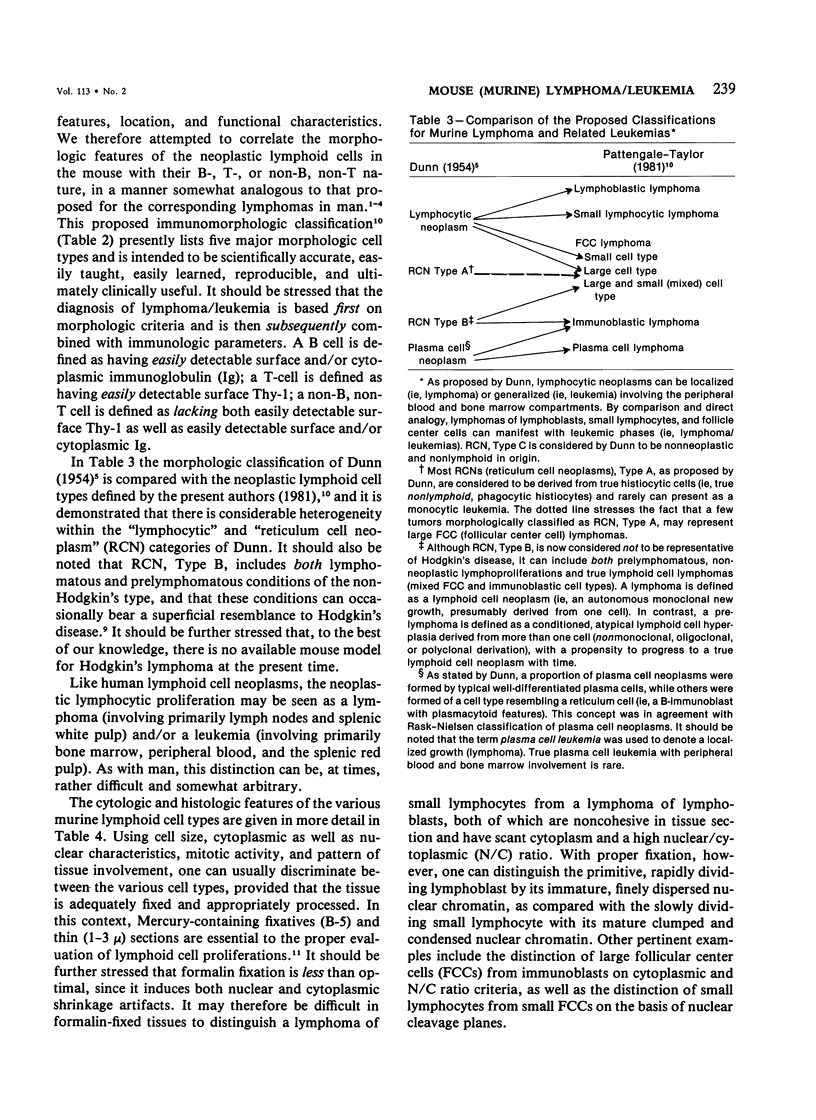
- Cantor H., Asofsky R., Talal N. Synergy among lymphoid cells mediating the graft-versus-host response. I. Synergy in graft-versus-host reactions produced by cells from NZB-Bl mice. J Exp Med. 1970 Feb;131(2):223–234. doi: 10.1084/jem.131.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., McVay-Boudreau L., Hugenberger J., Naidorf K., Shen F. W., Gershon R. K. Immunoregulatory circuits among T-cell sets. II. Physiologic role of feedback inhibition in vivo: absence in NZB mice. J Exp Med. 1978 Apr 1;147(4):1116–1125. doi: 10.1084/jem.147.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Lerman S. P., Thorbecke G. J. Properties of reticulum cell sarcomas in SJL/J mice. II. Fate of labeled tumor cells in normal and irradiated syngeneic mice. Cell Immunol. 1976 Apr;23(1):39–52. doi: 10.1016/0008-8749(76)90170-2. [DOI] [PubMed] [Google Scholar]
- Chused T. M., Steinberg A. D., Parker L. M. Enhanced antibody response of mice to polyinosinic-polycytidylic acid by antithymocyte serum and its age-dependent loss in NZB-W mice. J Immunol. 1973 Jul;111(1):52–57. [PubMed] [Google Scholar]
- Claesson M. H., Metcalf D. B lymphocyte colony-forming cells in the SJL/J mouse. J Immunol. 1977 Apr;118(4):1208–1212. [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz G., Hochman P. S. Do natural killer cells engage in regulated reactions against self to ensure homeostasis? Immunol Rev. 1979;44:13–41. doi: 10.1111/j.1600-065x.1979.tb00266.x. [DOI] [PubMed] [Google Scholar]
- DUNN T. B. Morphology and pathology of reticular neoplasms in the mouse: relationship to man. Ann N Y Acad Sci. 1958 Dec 5;76(3):619–629. doi: 10.1111/j.1749-6632.1958.tb54880.x. [DOI] [PubMed] [Google Scholar]
- DUNN T. B. Normal and pathologic anatomy of the reticular tissue in laboratory mice, with a classification and discussion of neoplasms. J Natl Cancer Inst. 1954 Jun;14(6):1281–1433. [PubMed] [Google Scholar]
- DUNN T. B. Plasma-cell neoplasms beginning in the ileocecal area in strain C3H mice. J Natl Cancer Inst. 1957 Sep;19(3):371–391. doi: 10.1093/jnci/19.3.371. [DOI] [PubMed] [Google Scholar]
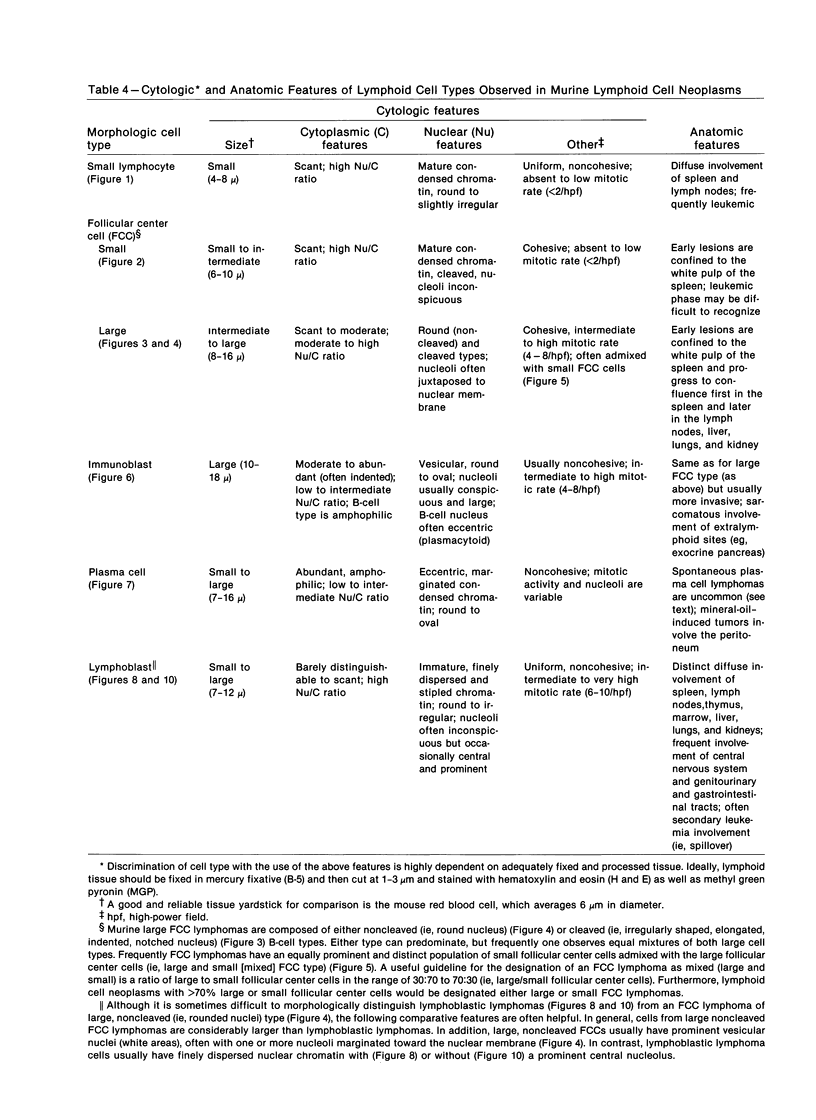
- Dalla-Favera R., Bregni M., Erikson J., Patterson D., Gallo R. C., Croce C. M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deringer M. K. Occurrence of mammary tumors, reticular neoplasms, and pulmonary tumors in strain BALB-cAnDe breeding female mice. J Natl Cancer Inst. 1965 Dec;35(6):1047–1052. [PubMed] [Google Scholar]
- Dunn T. B., Deringer M. K. Reticulum cell neoplasm, type B, or the "Hodgkin's-like lesion" of the mouse. J Natl Cancer Inst. 1968 Apr;40(4):771–821. [PubMed] [Google Scholar]
- East J. Immunopathology and neoplasms in New Zealand black (NZB) and SJL-J mice. Prog Exp Tumor Res. 1970;13:84–134. doi: 10.1159/000386038. [DOI] [PubMed] [Google Scholar]
- Epstein M. A. Epstein-Barr virus as the cause of a human cancer. Nature. 1978 Aug 24;274(5673):740–740. doi: 10.1038/274740a0. [DOI] [PubMed] [Google Scholar]
- Ford R. J., Ruppert B., Maizel A. L. SJL tumor: a neoplasm involving macrophages. Lab Invest. 1981 Aug;45(2):111–119. [PubMed] [Google Scholar]
- Foucar K., McKenna R. W., Frizzera G., Brunning R. D. Incidence and patterns of bone marrow and blood involvement by lymphoma in relationship to the Lukes-Collins classification. Blood. 1979 Dec;54(6):1417–1422. [PubMed] [Google Scholar]
- Frizzera G., Hanto D. W., Gajl-Peczalska K. J., Rosai J., McKenna R. W., Sibley R. K., Holahan K. P., Lindquist L. L. Polymorphic diffuse B-cell hyperplasias and lymphomas in renal transplant recipients. Cancer Res. 1981 Nov;41(11 Pt 1):4262–4279. [PubMed] [Google Scholar]
- Frizzera G., Moran E. M., Rappaport H. Angio-immunoblastic lymphadenopathy with dysproteinaemia. Lancet. 1974 Jun 1;1(7866):1070–1073. doi: 10.1016/s0140-6736(74)90553-4. [DOI] [PubMed] [Google Scholar]
- Fujiwara M., Cinader B. Cellular aspects of tolerance. V. The in vivo cooperative role of accessory and thymus derived cells in responsiveness and unresponsiveness of SJL mice. Cell Immunol. 1974 May;12(2):194–204. doi: 10.1016/0008-8749(74)90072-0. [DOI] [PubMed] [Google Scholar]
- Gelfand M. C., Steinberg A. D. Mechanism of allograft rejection in New Zealand mice. I. Cell synergy and its age-dependent loss. J Immunol. 1973 Jun;110(6):1652–1662. [PubMed] [Google Scholar]
- Giovanella B., Nilsson K., Zech L., Yim O., Klein G., Stehlin J. S. Growth of diploid, Epstein-Barr virus-carrying human lymphoblastoid cell lines heterotransplanted into nude mice under immunologically privileged conditions. Int J Cancer. 1979 Jul 15;24(1):103–113. doi: 10.1002/ijc.2910240118. [DOI] [PubMed] [Google Scholar]
- Gleichmann E., Gleichmann H., Wilke W. Autoimmunization and lymphomagenesis in parent to F1 combinations differing at the major histocompatibility complex: model for spontaneous disease caused by altered self-antigens? Transplant Rev. 1976;31:156–224. doi: 10.1111/j.1600-065x.1976.tb01454.x. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Warner N. L., Holmes M. C. Plasma-cell tumor induction in (NZB) x BALB/c)F1 hybrid mice. J Natl Cancer Inst. 1966 Aug;37(2):135–143. [PubMed] [Google Scholar]
- Gutman G. A., Warner N. L., Harris A. W. Immunoglobulin production by murine B-lymphoma cells. Clin Immunol Immunopathol. 1981 Feb;18(2):230–244. doi: 10.1016/0090-1229(81)90029-5. [DOI] [PubMed] [Google Scholar]
- Hanna M. G., Jr, Nettesheim P., Snodgrass M. J. Decreasing immune competence and development of reticulum cell sarcomas in lymphatic tissue of aged mice. J Natl Cancer Inst. 1971 Apr;46(4):809–824. [PubMed] [Google Scholar]
- Hanto D. W., Frizzera G., Gajl-Peczalska K. J., Sakamoto K., Purtilo D. T., Balfour H. H., Jr, Simmons R. L., Najarian J. S. Epstein-Barr virus-induced B-cell lymphoma after renal transplantation: acyclovir therapy and transition from polyclonal to monoclonal B-cell proliferation. N Engl J Med. 1982 Apr 15;306(15):913–918. doi: 10.1056/NEJM198204153061506. [DOI] [PubMed] [Google Scholar]
- Hardin J. A., Chused T. M., Steinberg A. D. Supressor cells in the graft vs host reaction. J Immunol. 1973 Aug;111(2):650–651. [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN H. S., BROWN M. B. Further observations on inhibition of lymphoid tumor development by shielding and partial-body irradiation of mice. J Natl Cancer Inst. 1951 Oct;12(2):427–436. [PubMed] [Google Scholar]
- Katz I. R., Asofsky R., Thorbecke G. J. Suppression of spontaneous reticulum cell sarcoma development and of syngeneic stimulator cell by anti-mu treatment of SJL/J mice. J Immunol. 1980 Sep;125(3):1355–1359. [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
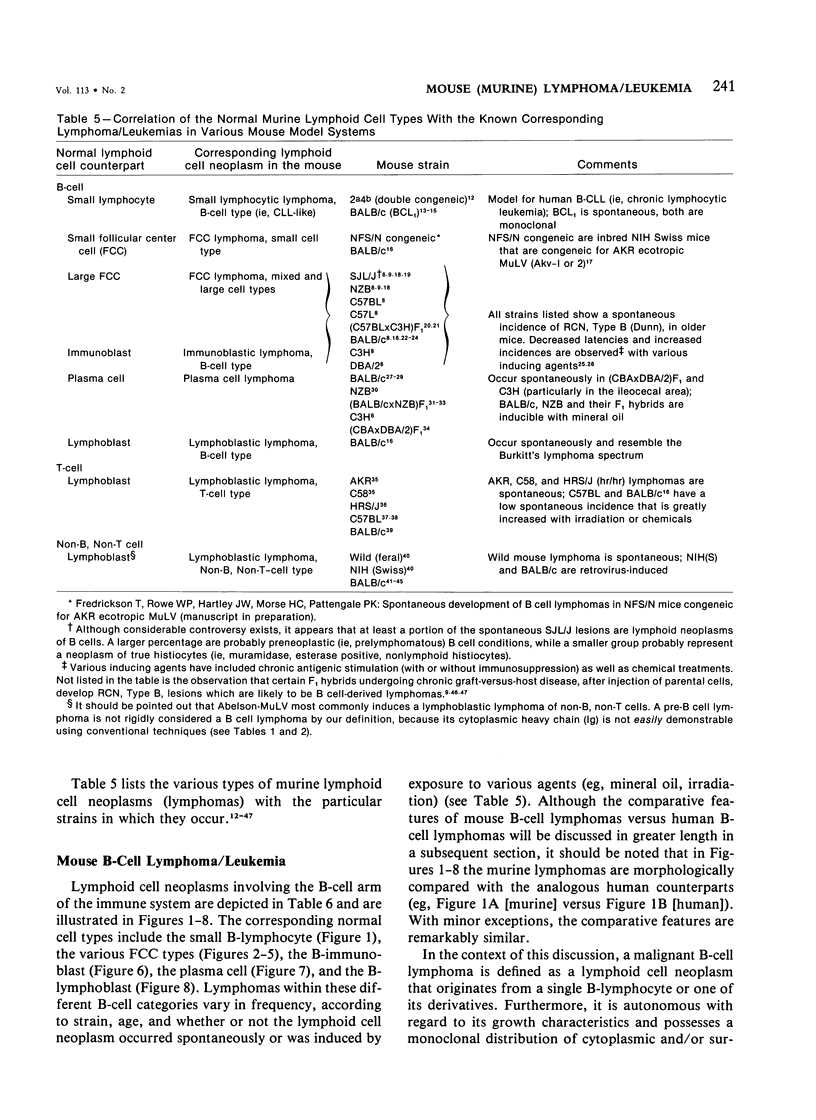
- Klassen L. W., Krakauer R. S., Steinberg A. D. Selective loss of suppressor cell function in New Zealand mice induced by NTA. J Immunol. 1977 Sep;119(3):830–830. [PubMed] [Google Scholar]
- Klein G. Lymphoma development in mice and humans: diversity of initiation is followed by convergent cytogenetic evolution. Proc Natl Acad Sci U S A. 1979 May;76(5):2442–2446. doi: 10.1073/pnas.76.5.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G. The Epstein-Barr virus and neoplasia. N Engl J Med. 1975 Dec 25;293(26):1353–1357. doi: 10.1056/NEJM197512252932607. [DOI] [PubMed] [Google Scholar]
- Klein G. The role of gene dosage and genetic transpositions in carcinogenesis. Nature. 1981 Nov 26;294(5839):313–318. doi: 10.1038/294313a0. [DOI] [PubMed] [Google Scholar]
- Krakauer R. S., Waldmann T. A., Strober W. Loss of suppressor T cells in adult NZB/NZW mice. J Exp Med. 1976 Sep 1;144(3):662–673. doi: 10.1084/jem.144.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger G. R., Malmgren R. A., Berard C. W. Malignant lymphomas and plasmacytosis in mice under prolonged immunosuppression and persistent antigenic stimulation. Transplantation. 1971 Feb;11(2):138–144. doi: 10.1097/00007890-197102000-00006. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Babcock G. F., Lynes M. A., Haughton G. Antigen-induced murine B-cell lymphomas. III. Passive anti-idiotype serum therapy and its combined effect with chemotherapy. J Natl Cancer Inst. 1979 Dec;63(6):1417–1422. [PubMed] [Google Scholar]
- Lanier L. L., Lynes M., Haughton G., Wettstein P. J. Novel type of murine B-cell lymphoma. Nature. 1978 Feb 9;271(5645):554–555. doi: 10.1038/271554a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Warner N. L., Ledbetter J. A., Herzenberg L. A. Quantitative immunofluorescent analysis of surface phenotypes of murine B cell lymphomas and plasmacytomas with monoclonal antibodies. J Immunol. 1981 Oct;127(4):1691–1697. [PubMed] [Google Scholar]
- Lerman S. P., Chapman J. M., Carswell E. A., Thorbecke G. J. Properties of reticulum-cell sarcomas in SJL/J mice. I. Proliferative response to tumor cells of T-derived lymphoid cells from normal mice. Int J Cancer. 1974 Dec 15;14(6):808–816. doi: 10.1002/ijc.2910140615. [DOI] [PubMed] [Google Scholar]
- Lukes R. J., Collins R. D. New approaches to the classification of the lymphomata. Br J Cancer Suppl. 1975 Mar;2:1–28. [PMC free article] [PubMed] [Google Scholar]
- Lukes R. J., Tindle B. H. Immunoblastic lymphadenopathy. A hyperimmune entity resembling Hodgkin's disease. N Engl J Med. 1975 Jan 2;292(1):1–8. doi: 10.1056/NEJM197501022920101. [DOI] [PubMed] [Google Scholar]
- MERWIN R. M., REDMON L. W. INDUCTION OF PLASMA CELL TUMORS AND SARCOMAS IN MICE BY DIFFUSION CHAMBERS PLACED IN THE PERITONEAL CAVITY. J Natl Cancer Inst. 1963 Oct;31:997–1017. [PubMed] [Google Scholar]
- METCALF D. Reticular tumours in mice subjected to prolonged antigenic stimulation. Br J Cancer. 1961 Dec;15:769–779. doi: 10.1038/bjc.1961.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K. B., Harris L. J., Stanton L. W., Erikson J., Watt R., Croce C. M. Transcriptionally active c-myc oncogene is contained within NIARD, a DNA sequence associated with chromosome translocations in B-cell neoplasia. Proc Natl Acad Sci U S A. 1983 Jan;80(2):519–523. doi: 10.1073/pnas.80.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieson B. J., Campbell P. S., Potter M., Asofsky R. Expression of Ly 1, Ly 2, Thy 1, and TL differentiation antigens on mouse T-cell tumors. J Exp Med. 1978 Apr 1;147(4):1267–1279. doi: 10.1084/jem.147.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire K. R., Law L. W. Abnormal serum immunoglobulins occurring with reticular neoplasms in a inbred strain of mouse. J Natl Cancer Inst. 1967 Dec;39(6):1197–1211. [PubMed] [Google Scholar]
- Meier H., Myers D. D., Huebner R. J. Genetic control by the hr-locus of susceptibility and resistance to leukemia. Proc Natl Acad Sci U S A. 1969 Jul;63(3):759–766. doi: 10.1073/pnas.63.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. D. Transplantation behavior of Hodgkin's-like reticulum cell neoplasms of strain SJL-J mice and results of tumor reinoculation. J Natl Cancer Inst. 1969 May;42(5):797–807. [PubMed] [Google Scholar]
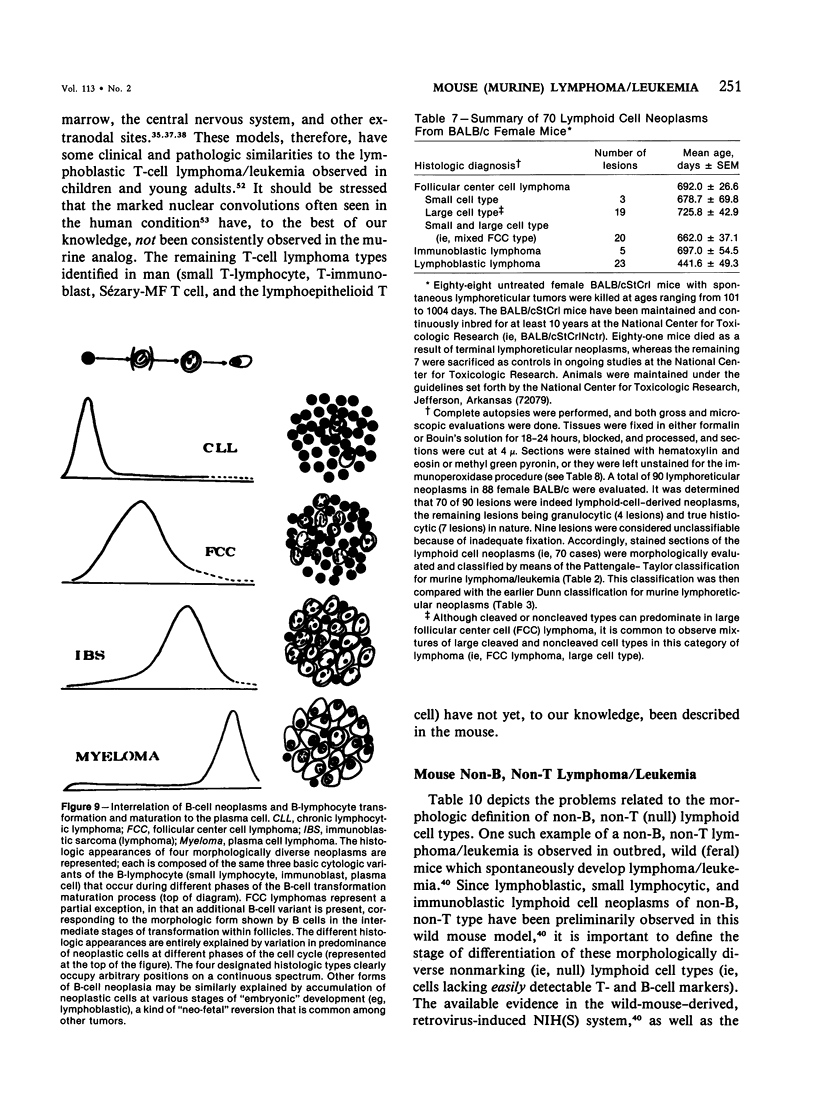
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Owens M. H., Bonavida B. Immune functions characteristic of SJL/J mice and their association with age and spontaneous reticulum cell sarcoma. Cancer Res. 1976 Mar;36(3):1077–1083. [PubMed] [Google Scholar]
- Owens M. H., Bonavida B. Initiation and characterization of cultured tumor lines from spontaneous reticulum cell sarcoma of SJL/J mice. Cancer Res. 1977 Dec;37(12):4439–4448. [PubMed] [Google Scholar]
- Panke T. W., Langlinais P. C., Vriend J., McCue M. J. An animal model for childhood convoluted T-cell lymphoma. Am J Pathol. 1978 Sep;92(3):595–610. [PMC free article] [PubMed] [Google Scholar]
- Pattengale P. K., Frith C. H. Immunomorphologic classification of spontaneous lymphoid cell neoplasms occurring in female BALB/c mice. J Natl Cancer Inst. 1983 Jan;70(1):169–179. [PubMed] [Google Scholar]
- Pattengale P. K., Smith R. W., Gerber P. B-cell characteristics of human peripheral and cord blood lymphocytes transformed by Epstein-Barr virus. J Natl Cancer Inst. 1974 Apr;52(4):1081–1086. doi: 10.1093/jnci/52.4.1081. [DOI] [PubMed] [Google Scholar]
- Pattengale P. K., Smith R. W., Gerber P. Selective transformation of B lymphocytes by E.B. virus. Lancet. 1973 Jul 14;2(7820):93–94. doi: 10.1016/s0140-6736(73)93286-8. [DOI] [PubMed] [Google Scholar]
- Pattengale P. K., Taylor C. R., Panke T., Tatter D., McCormick R. A., Rawlinson D. G., Davis R. L. Selective immunodeficiency and malignant lymphoma of the central nervous system. Possible relationship to the Epstein-Barr virus. Acta Neuropathol. 1979 Dec;48(3):165–169. doi: 10.1007/BF00690516. [DOI] [PubMed] [Google Scholar]
- Pattengale P. K., Taylor C. R., Pegalow C. Malignant B-cell lymphoma following and associated with infectious mononucleosis. A comparison of two cases. Am J Pediatr Hematol Oncol. 1981 Spring;3(1):35–42. [PubMed] [Google Scholar]
- Pattengale P. K., Taylor C. R., Twomey P., Hill S., Jonasson J., Beardsley T., Haas M. Immunopathology of B-cell lymphomas induced in C57BL/6 mice by dualtropic murine leukemia virus (MuLV). Am J Pathol. 1982 Jun;107(3):362–377. [PMC free article] [PubMed] [Google Scholar]
- Pollard M., Truitt R. L. Allogeneic bone marrow chimerism in germ-free mice. II. Prevention of reticulum cell sarcomas in SJL-J mice. Proc Soc Exp Biol Med. 1974 Feb;145(2):488–492. doi: 10.3181/00379727-145-37837. [DOI] [PubMed] [Google Scholar]
- Ponzio N. M., David C. S., Shreffler D. C., Thorbecke G. J. Properties of reticulum cell sarcomas in SJL/J mice. V. Nature of reticulum cell sarcoma surface antigen which induces proliferation of normal SJL/J T cells. J Exp Med. 1977 Jul 1;146(1):132–145. doi: 10.1084/jem.146.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portlock C. S., Rosenberg S. A. Chemotherapy of the non-Hodgkin's lymphomas: the Stanford experience. Cancer Treat Rep. 1977 Sep;61(6):1049–1055. [PubMed] [Google Scholar]
- Portlock C. S., Rosenberg S. A. No initial therapy for stage III and IV non-Hodgkin's lymphomas of favorable histologic types. Ann Intern Med. 1979 Jan;90(1):10–13. doi: 10.7326/0003-4819-90-1-10. [DOI] [PubMed] [Google Scholar]
- Potter M. Immunoglobulin-producing tumors and myeloma proteins of mice. Physiol Rev. 1972 Jul;52(3):631–719. doi: 10.1152/physrev.1972.52.3.631. [DOI] [PubMed] [Google Scholar]
- Potter M., Premkumar-Reddy E., Wivel N. A. Immunoglobulin production by lymphosarcomas induced by Abelson virus in mice. Natl Cancer Inst Monogr. 1978 May;(48):311–320. [PubMed] [Google Scholar]
- Purtilo D. T. Epstein-Barr-virus-induced oncogenesis in immune-deficient individuals. Lancet. 1980 Feb 9;1(8163):300–303. doi: 10.1016/s0140-6736(80)90792-8. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T., Hutt L., Bhawan J., Yang J. P., Cassel C., Allegra S., Rosen F. S. Immunodeficiency to the Epstein-Barr virus in the X-linked recessive lymphoproliferative syndrome. Clin Immunol Immunopathol. 1978 Feb;9(2):147–156. doi: 10.1016/0090-1229(78)90066-1. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T., Paquin L., DeFlorio D., Virzi F., Sakhuja R. Immunodiagnosis and immunopathogenesis of the X-linked recessive lymphoproliferative syndrome. Semin Hematol. 1979 Oct;16(4):309–343. [PubMed] [Google Scholar]
- Purtilo D. T., Szymanski I., Bhawan J., Yang J. P., Hutt L. M., Boto W., DeNicola L., Maier R., Thorley-Lawson D. Epstein-Barr virus infections in the X-linked recessive lymphoproliferative syndrome. Lancet. 1978 Apr 15;1(8068):798–801. doi: 10.1016/s0140-6736(78)92999-9. [DOI] [PubMed] [Google Scholar]
- Rabstein L. S., Gazdar A. F., Chopra H. C., Abelson H. T. Early morphological changes associated with infection by a murine nonthymic lymphatic tumor virus. J Natl Cancer Inst. 1971 Mar;46(3):481–491. [PubMed] [Google Scholar]
- Rask-Nielsen R., Ebbesen P. Spontaneous reticular neoplasms in (CBA X DBA/2)F1 mice, with special emphasis on the occurrence of plasma cell neoplasms. J Natl Cancer Inst. 1969 Sep;43(3):553–564. [PubMed] [Google Scholar]
- Raveché E. S., Steinberg A. D., Klassen L. W., Tjio J. H. Genetic studies in NZB mice. I. Spontaneous autoantibody production. J Exp Med. 1978 May 1;147(5):1487–1502. doi: 10.1084/jem.147.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Karre K., Kiessling R. Natural killer cells. Prog Allergy. 1981;28:66–159. [PubMed] [Google Scholar]
- Seibert K., Pollard M., Nordin A. Some aspects of humoral immunity in germ-free and conventional SJL-J mice in relation to age and pathology. Cancer Res. 1974 Jul;34(7):1707–1719. [PubMed] [Google Scholar]
- Seligmann M. Neoplasias and B-cell precursors. Nature. 1979 Jun 14;279(5714):578–578. doi: 10.1038/279578a0. [DOI] [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Siegler R., Rich M. A. Pathogenesis of reticulum cell sarcoma in mice. J Natl Cancer Inst. 1968 Jul;41(1):125–143. [PubMed] [Google Scholar]
- Siegler R., Zajdel S. Pathogenesis of Abelson-virus-induced murine leukemia. J Natl Cancer Inst. 1972 Jan;48(1):189–218. [PubMed] [Google Scholar]
- Slavin S., Strober S. Spontaneous murine B-cell leukaemia. Nature. 1978 Apr 13;272(5654):624–626. doi: 10.1038/272624a0. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Pasternak R. D. Syngeneic mixed lymphocyte reaction in mice: strain distribution, kinetics, participating cells, and absence in NZB mice. J Immunol. 1978 Nov;121(5):1889–1892. [PubMed] [Google Scholar]
- Steinberg A. D., Law L. D., Talal N. The role of NZB-NZW F1 thymus in experimental tolerance and auto-immunity. Arthritis Rheum. 1970 Jul-Aug;13(4):369–377. doi: 10.1002/art.1780130402. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L., Byron K. S., Brewster F. E., Purtilo D. T. Deficient natural killer cell activity in x-linked lymphoproliferative syndrome. Science. 1980 Oct 31;210(4469):543–545. doi: 10.1126/science.6158759. [DOI] [PubMed] [Google Scholar]
- Talal N., Steinberg A. D. The pathogenesis of autoimmunity in New Zealand black mice. Curr Top Microbiol Immunol. 1974;64(0):79–103. doi: 10.1007/978-3-642-65848-8_3. [DOI] [PubMed] [Google Scholar]
- Taub R., Kirsch I., Morton C., Lenoir G., Swan D., Tronick S., Aaronson S., Leder P. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. R. Classification of lymphoma. Arch Pathol Lab Med. 1978 Nov;102(11):549–554. [PubMed] [Google Scholar]
- Taylor C. R. Immuno-histological observations upon the development of reticulum cell sarcoma in the mouse. J Pathol. 1976 Apr;118(4):201–219. doi: 10.1002/path.1711180403. [DOI] [PubMed] [Google Scholar]
- Truitt R. L., Pollard M. Allogeneic bone marrow chimerism in germ-free mice. IV. Therapy of "Hodgkin's-like" reticulum cell sarcoma in SJL mice. Transplantation. 1976 Jan;21(1):12–16. doi: 10.1097/00007890-197601000-00003. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Yuan D., Krolick K., Isakson P., Knapp M., Slavin S., Strober S. Characterization of the spontaneous murine B cell leukemia (BCL1). III. Evidence for monoclonality by using an anti-idiotype antibody. J Immunol. 1979 May;122(5):1649–1654. [PubMed] [Google Scholar]
- Vogler L. B., Preud'homme J. L., Seligmann M., Gathings W. E., Crist W. M., Cooper M. D., Bollum F. J. Diversity of immunoglobulin expression in leukaemic cells resembling B-lymphocyte precursors. Nature. 1981 Mar 26;290(5804):339–341. doi: 10.1038/290339a0. [DOI] [PubMed] [Google Scholar]
- Wanebo H. J., Gallmeier W. M., Boyse E. A., Old L. J. Paraproteinemia and reticulum cell sarcoma in an inbred mouse strain. Science. 1966 Nov 18;154(3751):901–903. doi: 10.1126/science.154.3751.901. [DOI] [PubMed] [Google Scholar]
- Warner N. L. Neoplasms of immunoglobulin-producing cells in mice. Recent Results Cancer Res. 1978;64:316–324. doi: 10.1007/978-3-642-81246-0_37. [DOI] [PubMed] [Google Scholar]
- Warnke R. A., Slavin S., Coffman R. L., Butcher E. C., Knapp M. R., Strober S., Weissman I. L. The pathology and homing of a transplantable murine B cell leukemia (BCL1). J Immunol. 1979 Sep;123(3):1181–1188. [PubMed] [Google Scholar]
- Watanabe N., Kojima S., Shen F. W., Ovary Z. Suppression of IgE antibody production in SJL mice. II. Expression of Ly-1 antigen on helper and nonspecific suppressor T cells. J Immunol. 1977 Feb;118(2):485–488. [PubMed] [Google Scholar]
- Watanabe N., Ovary Z. Suppression of IgE antibody production in SJL mice. III. Characterization of a suppressor substance extracted from normal SJL spleen cells. J Exp Med. 1977 Jun 1;145(6):1501–1510. doi: 10.1084/jem.145.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur S. M., Bonavida B. Expression of hybrid Ia molecules on the cell surface of reticulum cell sarcomas that are undetectable on host SJL/J lymphocytes. J Exp Med. 1981 Mar 1;153(3):501–513. doi: 10.1084/jem.153.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerganian G., Gagnon H. J., Battaglino A. Angioimmunoblastic lymphadenopathy, immunoblastic sarcoma of B cells. Animal model: autoimmune-prone inbred Armenian hamster. Am J Pathol. 1978 Apr;91(1):209–212. [PMC free article] [PubMed] [Google Scholar]
- Ziegler J. L., Drew W. L., Miner R. C., Mintz L., Rosenbaum E., Gershow J., Lennette E. T., Greenspan J., Shillitoe E., Beckstead J. Outbreak of Burkitt's-like lymphoma in homosexual men. Lancet. 1982 Sep 18;2(8299):631–633. doi: 10.1016/s0140-6736(82)92740-4. [DOI] [PubMed] [Google Scholar]