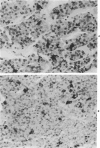
Abstract
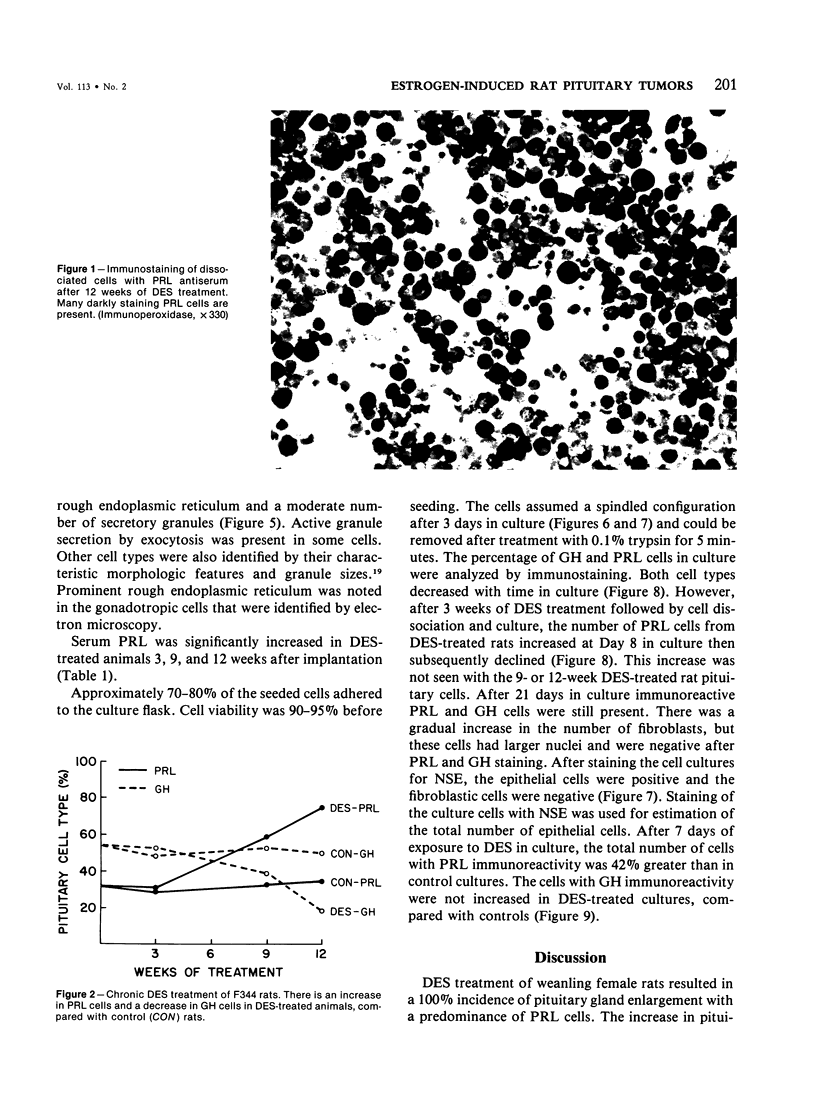
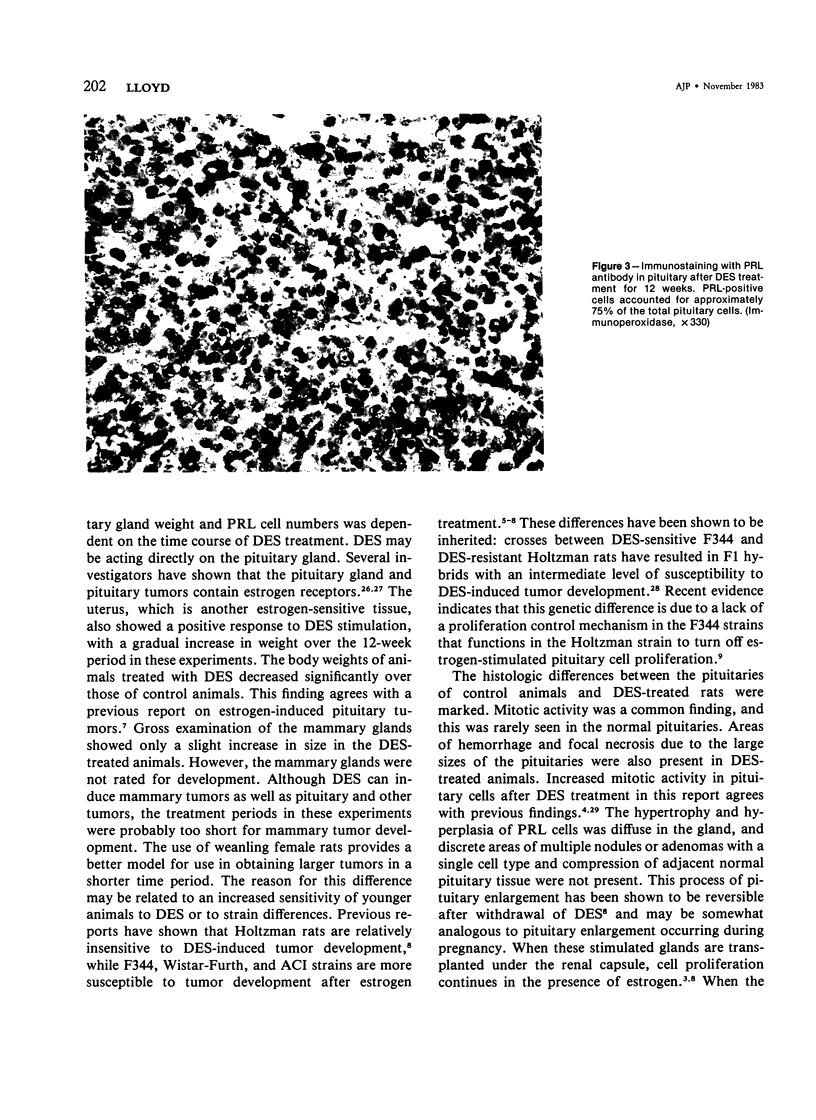
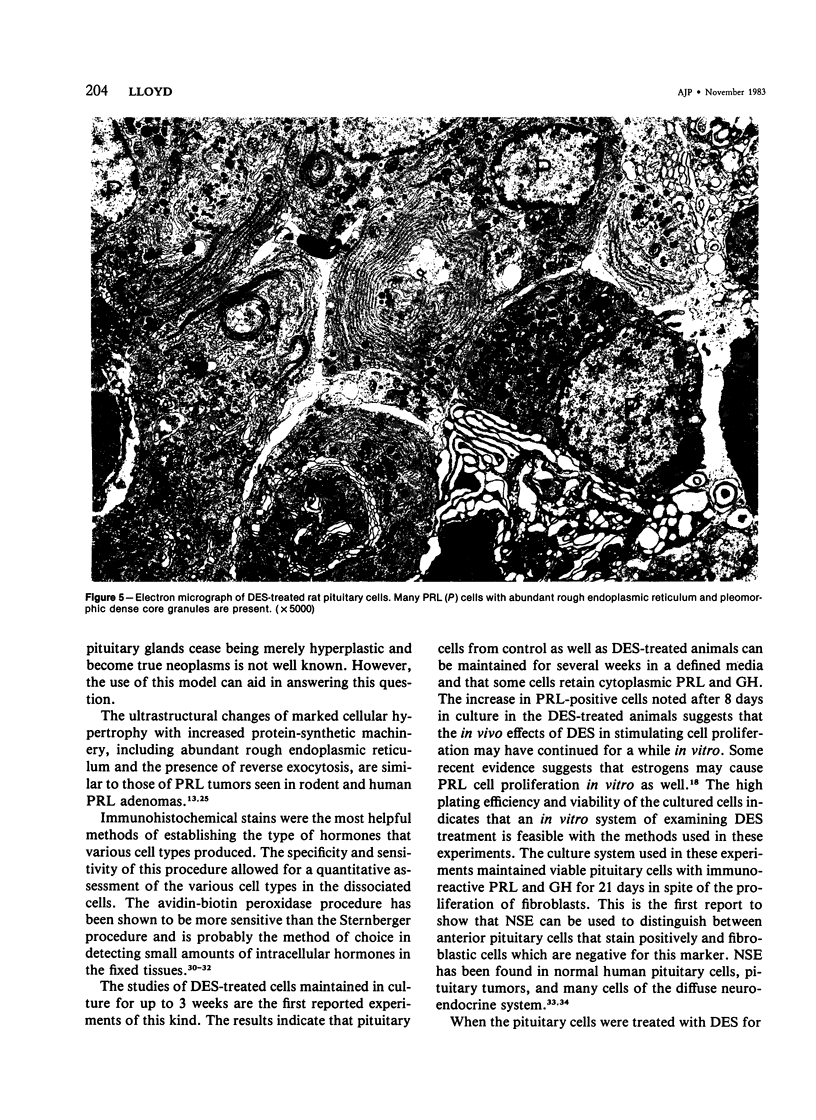
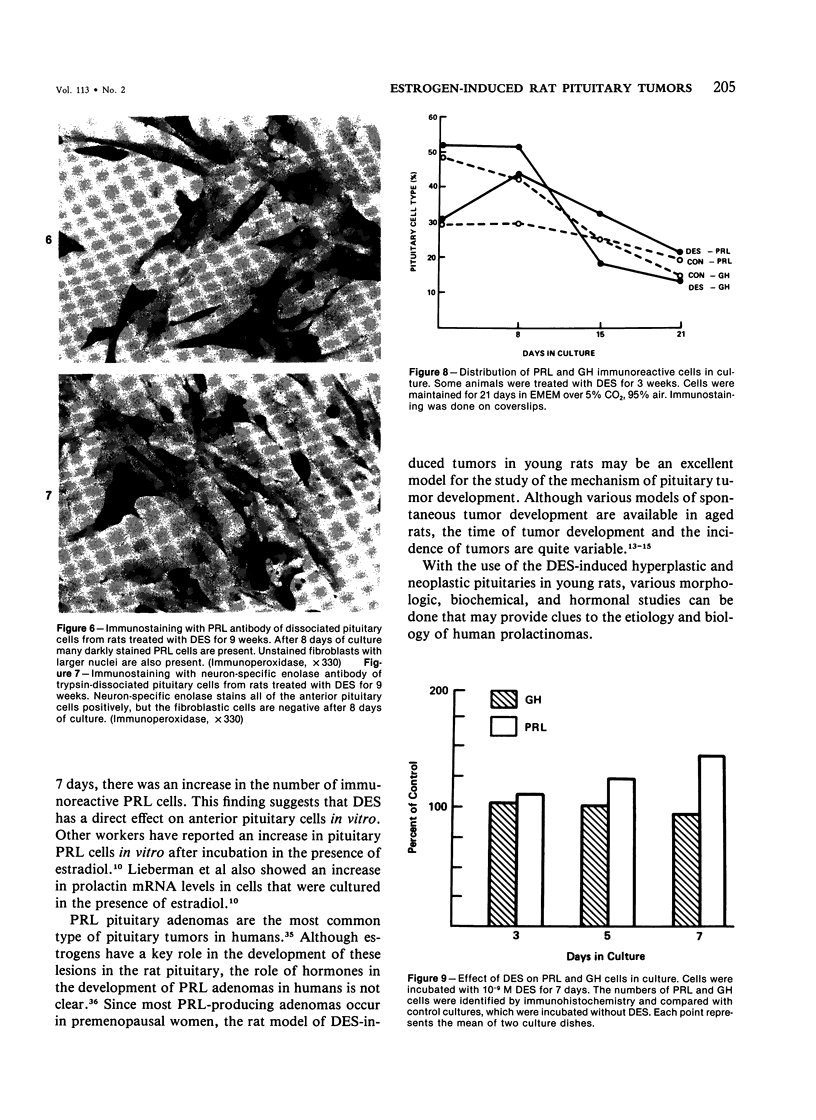
Diethylstilbestrol (DES) treatment of weanling F344 female rats resulted in enlarged pituitary glands and diffuse pituitary prolactin (PRL) cell hyperplasia in all animals after 9 and 12 weeks of treatment. Serum PRL was significantly greater than in control rats (P less than 0.001). Immunohistochemical studies showed that most of the pituitary gland cells consisted of PRL cells. Ultrastructural studies showed increased numbers of PRL cells with hyperplasia of the rough endoplasmic reticulum and decreased numbers of secretory granules. There was a decrease in the relative number of growth hormone (GH) and other cell types in the anterior pituitary. Pituitary tumors and normal pituitary glands were dissociated with trypsin and maintained in culture for 3 weeks. The numbers of PRL and GH cells decreased with time in both groups, and there was an increase in the number of fibroblasts. Staining of the culture cells with neuron-specific enolase showed that the anterior pituitary cells were positive for this enzyme, while the fibroblastic cells were negative. When dissociated pituitary cells were cultured in the presence of 10(-9) M DES for 7 days, there was a 42% increase in the number of immunoreactive PRL cells. These results indicate that DES-treated rats provide an excellent model for study of the in vivo and in vitro regulation of pituitary hyperplasia and neoplasia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bethea C. L., Weiner R. I. Human prolactin secreting adenoma cells maintained on extracellular matrix. Endocrinology. 1981 Jan;108(1):357–360. doi: 10.1210/endo-108-1-357. [DOI] [PubMed] [Google Scholar]
- CLIFTON K. H., MEYER R. K. Mechanism of anterior pituitary tumor induction by estrogen. Anat Rec. 1956 May;125(1):65–81. doi: 10.1002/ar.1091250106. [DOI] [PubMed] [Google Scholar]
- Childs G., Unabia G. Application of the avidin-biotin-peroxidase complex (ABC) method to the light microscopic localization of pituitary hormones. J Histochem Cytochem. 1982 Jul;30(7):713–716. doi: 10.1177/30.7.6286753. [DOI] [PubMed] [Google Scholar]
- De Nicola A. F., von Lawzewitsch I., Kaplan S. E., Libertun C. Biochemical and ultrastructural studies on estrogen-induced pituitary tumors in F344 rats. J Natl Cancer Inst. 1978 Sep;61(3):753–763. [PubMed] [Google Scholar]
- Duello T. M., Halmi N. S. Immunocytochemistry of prolactin-producing human pituitary adenomas. Am J Anat. 1980 Aug;158(4):463–469. doi: 10.1002/aja.1001580408. [DOI] [PubMed] [Google Scholar]
- Holtzman S., Stone J. P., Shellabarger C. J. Influence of diethylstilbestrol treatment on prolactin cells of female ACI and Sprague-Dawley rats. Cancer Res. 1979 Mar;39(3):779–784. [PubMed] [Google Scholar]
- Horwitz K. B., Costlow M. E., McGuire W. L. MCF-7; a human breast cancer cell line with estrogen, androgen, progesterone, and glucocorticoid receptors. Steroids. 1975 Dec;26(6):785–795. doi: 10.1016/0039-128x(75)90110-5. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kovacs K., Horvath E., Ezrin C. Pituitary adenomas. Pathol Annu. 1977;12(Pt 2):341–382. [PubMed] [Google Scholar]
- Kovacs K., Ilse G., Ryan N., McComb D. J., Horvath E., Chen H. J., Walfish P. G. Pituitary prolactin cell hyperplasia. Horm Res. 1980;12(2):87–95. doi: 10.1159/000179108. [DOI] [PubMed] [Google Scholar]
- Leavitt W. W., Kimmel G. L., Friend J. P. Steroid hormone uptake by anterior pituitary cell suspensions. Endocrinology. 1973 Jan;92(1):94–103. doi: 10.1210/endo-92-1-94. [DOI] [PubMed] [Google Scholar]
- Lee A. K., DeLellis R. A., Blount M., Nunnemacher G., Wolfe H. J. Pituitary proliferative lesions in aging male Long-Evans rats. A model of mixed multiple endocrine neoplasia syndrome. Lab Invest. 1982 Dec;47(6):595–602. [PubMed] [Google Scholar]
- Lieberman M. E., Maurer R. A., Claude P., Gorski J. Prolactin synthesis in primary cultures of pituitary cells: regulation by estradiol. Mol Cell Endocrinol. 1982 Mar;25(3):277–294. doi: 10.1016/0303-7207(82)90084-3. [DOI] [PubMed] [Google Scholar]
- Lloyd H. M., Meares J. D., Jacobi J. Effects of oestrogen and bromocryptine on in vivo secretion and mitosis in prolactin cells. Nature. 1975 Jun 5;255(5508):497–498. doi: 10.1038/255497a0. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Fruhman J. Comparison of peroxidase-antiperoxidase and avidin-biotin complex methods with radioimmunoassay antibodies. Am J Clin Pathol. 1982 Nov;78(5):795–796. doi: 10.1093/ajcp/78.5.795. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Gikas P. W., Chandler W. F. Prolactin and growth hormone-producing pituitary adenomas. An immunohistochemical and ultrastructural study. Am J Surg Pathol. 1983 Apr;7(3):251–260. doi: 10.1097/00000478-198304000-00004. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., McShan W. H. Effects of LH-RH on isolated pituitary gonadotropic cells. Proc Soc Exp Biol Med. 1976 Jan;151(1):160–162. doi: 10.3181/00379727-151-39165. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., McShan W. H. Study of rat anterior pituitary cells separated by velocity sedimentation at unit gravity. Endocrinology. 1973 Jun;92(6):1639–1651. doi: 10.1210/endo-92-6-1639. [DOI] [PubMed] [Google Scholar]
- Nakano H., Fawcett C. P., McCann S. M. Enzymatic dissociation and short-term culture of isolated rat anterior pituitary cells for studies on the control of hormone secretion. Endocrinology. 1976 Feb;98(2):278–288. doi: 10.1210/endo-98-2-278. [DOI] [PubMed] [Google Scholar]
- Schmechel D., Marangos P. J., Brightman M. Neurone-specific enolase is a molecular marker for peripheral and central neuroendocrine cells. Nature. 1978 Dec 21;276(5690):834–836. doi: 10.1038/276834a0. [DOI] [PubMed] [Google Scholar]
- Snyder J., Hymer W. C., Wilfinger W. W. Culture of human pituitary prolactin and growth hormone cells. Cell Tissue Res. 1978 Aug 16;191(3):379–388. doi: 10.1007/BF00219803. [DOI] [PubMed] [Google Scholar]
- Sorrentino J. M., Kirkland W. L., Sirbasku D. A. Control of cell growth. I. Estrogen-dependent growth in vivo of a rat pituitary tumor cell line. J Natl Cancer Inst. 1976 Jun;56(6):1149–1154. doi: 10.1093/jnci/56.6.1149. [DOI] [PubMed] [Google Scholar]
- Sorrentino J. M., Kirkland W. L., Sirbasku D. A. Control of cell growth. II. Requirement of thyroid hormones for the in vivo estrogen-dependent growth of rat pituitary tumor cells. J Natl Cancer Inst. 1976 Jun;56(6):1155–1158. doi: 10.1093/jnci/56.6.1155. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Sonnenschein C. Estrogen receptor levels in estrogen sensitive cells in culture. J Steroid Biochem. 1979 Aug;11(2):1185–1190. doi: 10.1016/0022-4731(79)90172-9. [DOI] [PubMed] [Google Scholar]
- Stone J. P., Holtzman S., Shellabarger C. J. Neoplastic responses and correlated plasma prolactin levels in diethylstilbestrol-treated ACI and Sprague-Dawley rats. Cancer Res. 1979 Mar;39(3):773–778. [PubMed] [Google Scholar]
- THOMPSON S. W., HUNT R. D. SPONTANEOUS TUMORS IN THE SPRAGUE-DAWLEY RAT: INCIDENCE RATES OF SOME TYPES OF NEOPLASMS AS DETERMINED BY SERIAL SECTION VERSUS SINGLE SECTION TECHNICS. Ann N Y Acad Sci. 1963 Nov 4;108:833–845. doi: 10.1111/j.1749-6632.1963.tb13422.x. [DOI] [PubMed] [Google Scholar]
- Trouillas J., Girod C., Claustrat B., Curé M., Dubois M. P. Spontaneous pituitary tumors in the Wistar/Furth/Ico rat strain. An animal model of human prolactin adenoma. Am J Pathol. 1982 Oct;109(1):57–70. [PMC free article] [PubMed] [Google Scholar]
- Wiklund J. A., Gorski J. Genetic differences in estrogen-induced deoxyribonucleic acid synthesis in the rat pituitary: correlations with pituitary tumor susceptibility. Endocrinology. 1982 Oct;111(4):1140–1149. doi: 10.1210/endo-111-4-1140. [DOI] [PubMed] [Google Scholar]
- Wiklund J., Rutledge J., Gorski J. A genetic model for the inheritance of pituitary tumor susceptibility in F344 rats. Endocrinology. 1981 Nov;109(5):1708–1714. doi: 10.1210/endo-109-5-1708. [DOI] [PubMed] [Google Scholar]
- Wiklund J., Wertz N., Gorski J. A comparison of estrogen effects on uterine and pituitary growth and prolactin synthesis in F344 and Holtzman rats. Endocrinology. 1981 Nov;109(5):1700–1707. doi: 10.1210/endo-109-5-1700. [DOI] [PubMed] [Google Scholar]