Abstract
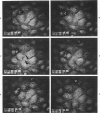
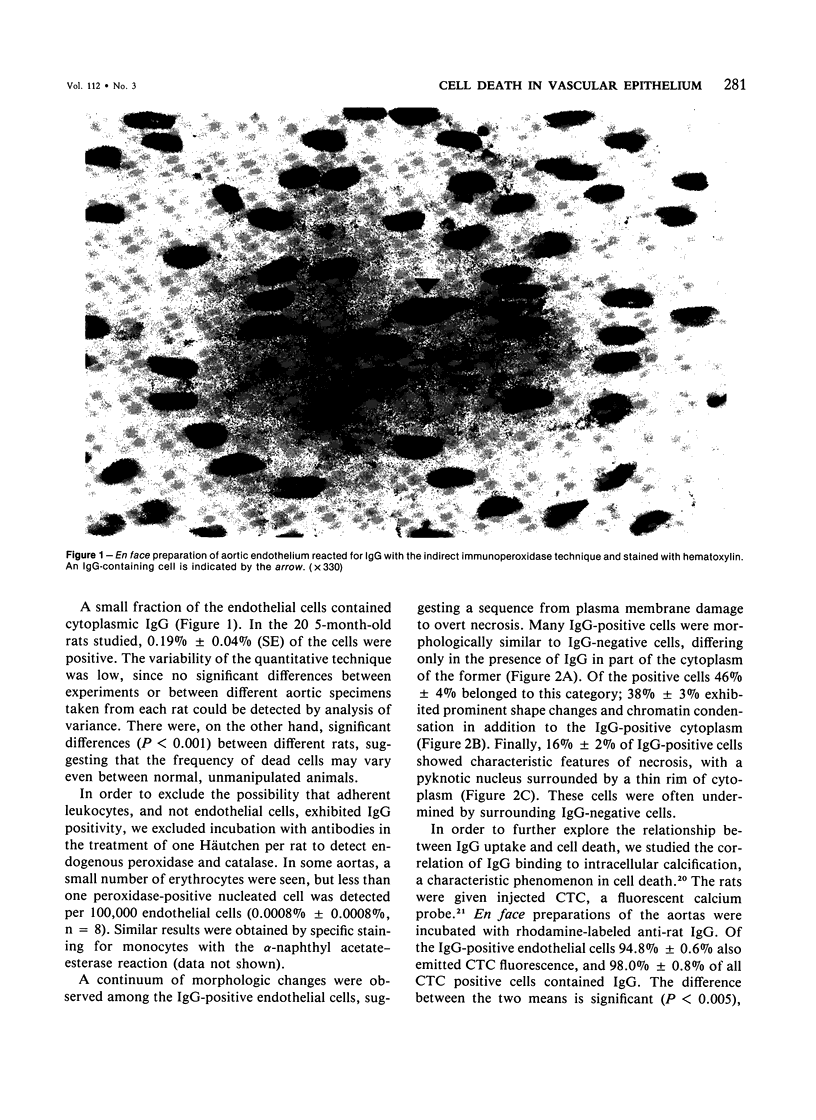
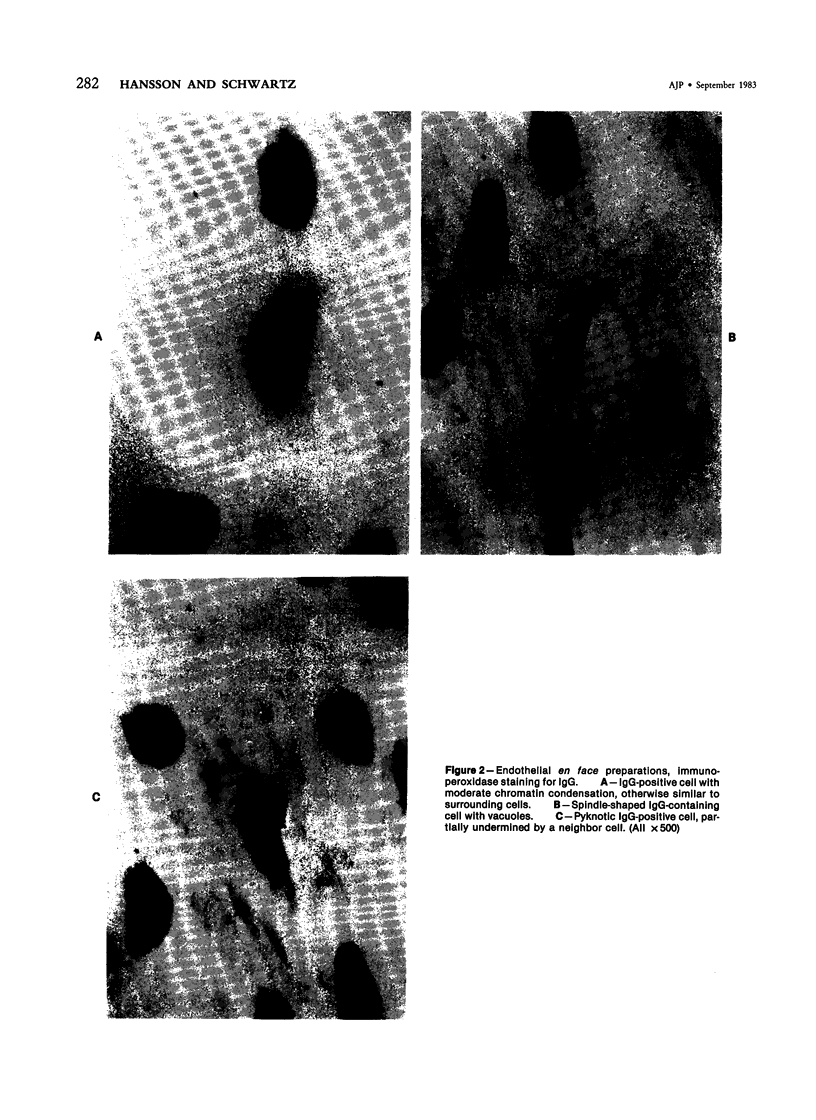
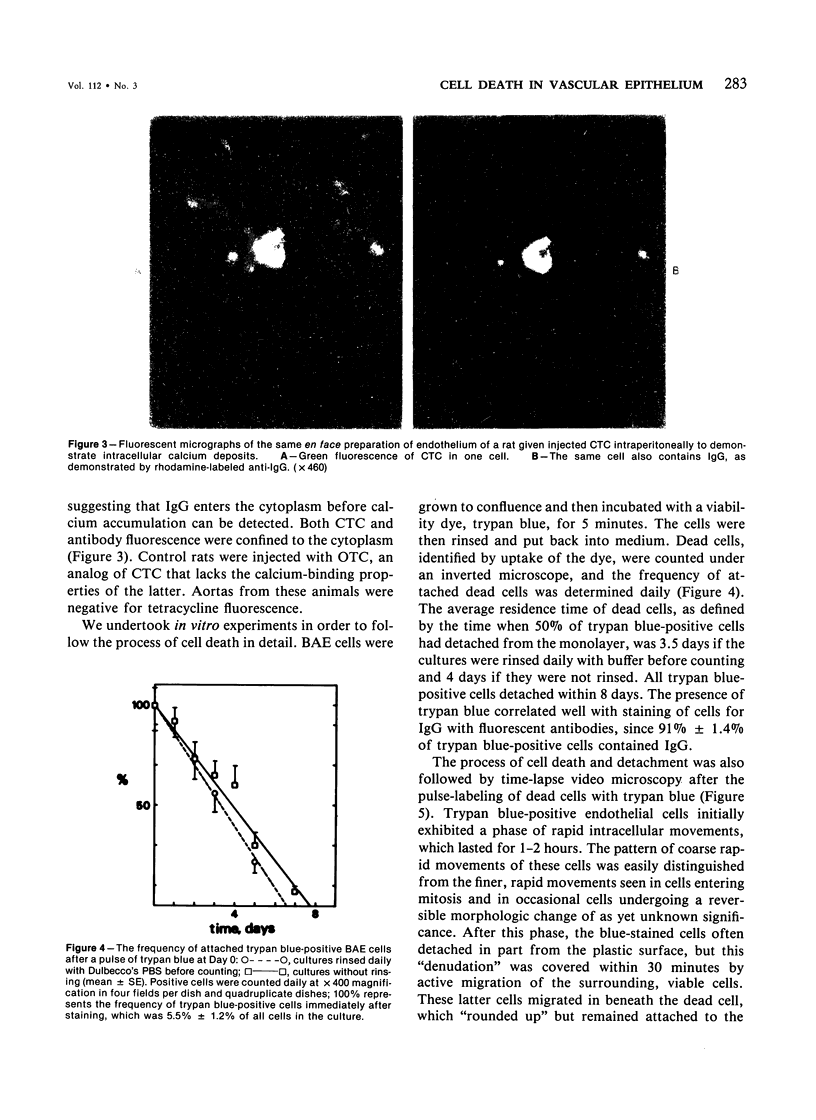
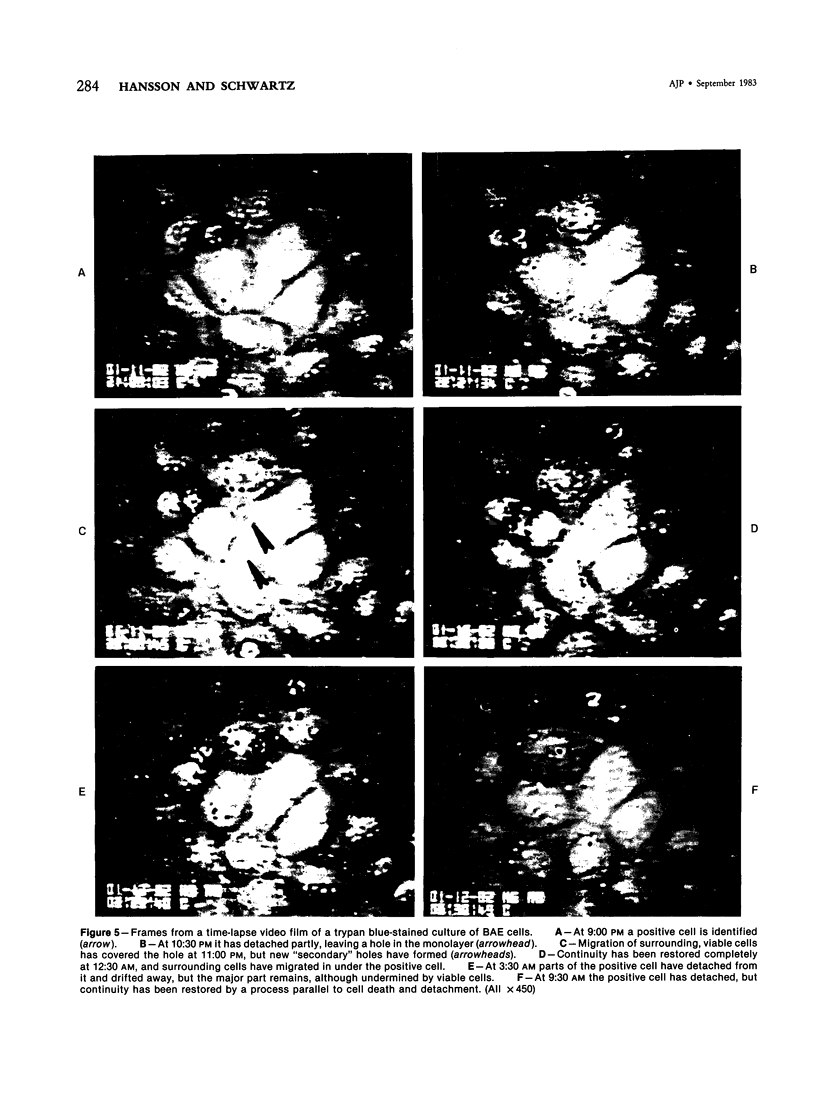
Focal, spontaneous cell death in the rat aortic endothelium was demonstrated by cytochemistry. Cells with intracellular calcium deposits, indicating cell death with mitochondrial calcification, were identified by chlorotetracycline fluorescence. The same cells also contained cytoplasmic IgG, which binds to cytoskeletal components of the dead cell. The immunocytochemical detection of IgG in en face preparations was used as a quantitative method for detecting cell death in the aortic endothelium. The use of an indirect immunoperoxidase technique and "Häutchens" of paraformaldehyde-fixed tissue provided high sensitivity and cellular recovery with low background. A cell death frequency of 0.19% +/- 0.04% was observed in 5-month-old Sprague-Dawley rats. When compared with the replication rate of aortic endothelium in these animals, the data suggest that dead cells remain in the endothelium for more than 24 hours. This conclusion was supported by in vitro studies. Confluent cultures of bovine aortic endothelium were pulsed with trypan blue, and the residence time of blue cells was 3.5-4 days in non-flow culture system. Time-lapse video microscopy showed a prolonged cell death process with a phase of rapid intracellular movements, followed by undermining by surrounding cells and fragmentation of the dead cell. Migration of surrounding cells rapidly covered partial detachments of the dead cell, so that no holes could be detected in the monolayer when the dead cell finally detached. It is concluded that the normal turnover of cells in the aortic endothelium involves a prolonged phase of in situ cell death and finally detachment with very little or no exposure of subendothelial structures.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkerud S., Bondjers G. Endothelial integrity and viability in the aorta of the normal rabbit and rat as evaluated with dye exclusion tests and interference contrast microscopy. Atherosclerosis. 1972 May-Jun;15(3):285–300. doi: 10.1016/0021-9150(72)90019-6. [DOI] [PubMed] [Google Scholar]
- Caplan B. A., Schwartz C. J. Increased endothelial cell turnover in areas of in vivo Evans Blue uptake in the pig aorta. Atherosclerosis. 1973 May-Jun;17(3):401–417. doi: 10.1016/0021-9150(73)90031-2. [DOI] [PubMed] [Google Scholar]
- Constantinides P., Robinson M. Ultrastructural injury of arterial endothelium. 1. Effects of pH, osmolarity, anoxia, and temperature. Arch Pathol. 1969 Aug;88(2):99–105. [PubMed] [Google Scholar]
- Farber J. L., Chien K. R., Mittnacht S., Jr Myocardial ischemia: the pathogenesis of irreversible cell injury in ischemia. Am J Pathol. 1981 Feb;102(2):271–281. [PMC free article] [PubMed] [Google Scholar]
- Gaynor E. Increased mitotic activity in rabbit endothelium after endotoxin. An autoradiographic study. Lab Invest. 1971 Apr;24(4):318–320. [PubMed] [Google Scholar]
- Gerrity R. G., Richardson M., Somer J. B., Bell F. P., Schwartz C. J. Endothelial cell morphology in areas of in vivo Evans blue uptake in the aorta of young pigs. II. Ultrastructure of the intima in areas of differing permeability to proteins. Am J Pathol. 1977 Nov;89(2):313–334. [PMC free article] [PubMed] [Google Scholar]
- Glenn K. C., Ross R. Human monocyte-derived growth factor(s) for mesenchymal cells: activation of secretion by endotoxin and concanavalin A. Cell. 1981 Sep;25(3):603–615. doi: 10.1016/0092-8674(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Björnheden T., Bylock A., Bondjers G. Fc-dependent binding of monocytes to areas with endothelial injury in the rabbit aorta. Exp Mol Pathol. 1981 Jun;34(3):264–280. doi: 10.1016/0014-4800(81)90044-7. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Bondjers G., Bylock A., Hjalmarsson L. Ultrastructural studies on the localization of IgG in the aortic endothelium and subendothelial intima of atherosclerotic and nonatherosclerotic rabbits. Exp Mol Pathol. 1980 Dec;33(3):302–315. doi: 10.1016/0014-4800(80)90028-3. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Bondjers G. Endothelial proliferation and atherogenesis in rabbits with moderate hypercholesterolemia. Artery. 1980;7(4):316–329. [PubMed] [Google Scholar]
- Hansson G. K., Bondjers G., Nilsson L. A. Plasma protein accumulation in injured endothelial cells. Immunofluorescent localization of IgG and fibrinogen in the rabbit aortic endothelium. Exp Mol Pathol. 1979 Feb;30(1):12–26. doi: 10.1016/0014-4800(79)90078-9. [DOI] [PubMed] [Google Scholar]
- Inoué S. Video image processing greatly enhances contrast, quality, and speed in polarization-based microscopy. J Cell Biol. 1981 May;89(2):346–356. doi: 10.1083/jcb.89.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennette D. A. An improved mounting medium for immunofluorescence microscopy. Am J Clin Pathol. 1978 Jun;69(6):647–648. doi: 10.1093/ajcp/69.6.647. [DOI] [PubMed] [Google Scholar]
- Majno G., Joris I. Endothelium 1977: a review. Adv Exp Med Biol. 1978;104:169-225, 481-526. doi: 10.1007/978-1-4684-7787-0_9. [DOI] [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial injury and regeneration. IV. Endotoxin: a nondenuding injury to aortic endothelium. Lab Invest. 1983 Jan;48(1):25–34. [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial regeneration. III. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981 Apr;44(4):301–308. [PubMed] [Google Scholar]
- Schwartz S. M., Benditt E. P. Aortic endothelial cell replication. I. Effects of age and hypertension in the rat. Circ Res. 1977 Aug;41(2):248–255. doi: 10.1161/01.res.41.2.248. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Benditt E. P. Cell replication in the aortic endothelium: a new method for study of the problem. Lab Invest. 1973 Jun;28(6):699–707. [PubMed] [Google Scholar]
- Schwartz S. M., Benditt E. P. Clustering of replicating cells in aortic endothelium. Proc Natl Acad Sci U S A. 1976 Feb;73(2):651–653. doi: 10.1073/pnas.73.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M., Gajdusek C. M., Selden S. C., 3rd Vascular wall growth control: the role of the endothelium. Arteriosclerosis. 1981 Mar-Apr;1(2):107–126. doi: 10.1161/01.atv.1.2.107. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M. Selection and characterization of bovine aortic endothelial cells. In Vitro. 1978 Dec;14(12):966–980. doi: 10.1007/BF02616210. [DOI] [PubMed] [Google Scholar]
- TAPP E., CARROLL R., KOVACS K. TETRACYCLINE FLUORESCENCE IN EXPERIMENTAL RENAL LESIONS. Arch Pathol. 1965 Jun;79:629–634. [PubMed] [Google Scholar]
- Warren B. A. A method for the production of "en face" preparations one cell in thickness. J R Microsc Soc. 1965 Dec;85(4):407–413. doi: 10.1111/j.1365-2818.1965.tb02141.x. [DOI] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]