Abstract
Objective
We evaluated the effects of different levels of the potent environmental toxicant and teratogen, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), on molar development in mice in 6 inbred strains, all with TCDD responsive Ahr alleles.
Design
Pregnant females were exposed on gestation day 13 to four different levels of TCDD (control, 0.01, 0.1, and 1.0 μg/kg) and their offspring were examined for the frequency of missing third molars (M3s) and for differences in first mandibular molar (M1) cuspal morphology.
Results
Missing M3s were prevalent only in mice in two strains, C3H/HeJ and CBA/J, and their frequency significantly increased with increasing TCDD exposure. The frequency of the M1 variant was high in mice in only one strain, C57BL/10J, and was significantly higher in the treated compared with the control group.
Conclusions
Inbred mice strains exhibited differential responses to TCDD suggesting that there is a genetic component, beyond Ahr differences, mediating the effects of TCDD on molar development.
Introduction
Embryonic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a potent environmental toxicant, has been shown to interfere with tooth development. In humans, increased incidences of dental defects have been associated with childhood TCDD exposure.1 In addition, in vitro studies have found that TCDD exposure alters dental cell organization, enamel and dentin deposition, and cuspal morphology in cultured embryonic molar teeth.2,3 Similarly, the continuously-erupting incisors of rats exposed to TCDD from 10 to 20 weeks of age exhibited dose-dependent changes in dental tissues.4
While tooth development, in general, appears to be sensitive to TCDD’s effects, strain-specific differences in sensitivity have been attributed primarily to differences in the TCDD binding affinity of different alleles at the aryl hydrocarbon receptor (AHR) locus.5,6 We therefore began an investigation into the effects of varying prenatal exposures of TCDD on molar size and shape in mice from six different inbred strains, all with high affinity Ahr alleles. During the molar digitization process, we discovered a number of mice with missing third molars as well as some mice with an unusual morphological variant of the first mandibular molar (M1). We decided to test whether prenatal exposure to TCDD might be responsible for these effects and if so, whether the effect of TCDD on these characters depended on strain. This paper reports the results of that investigation.
Materials and methods
Population
Six inbred mouse strains (C57BL/6J, BALB/cByJ, A/J, CBA/J, C3H/HeJ, and C57BL/10J) possessing the high affinity ligand binding Ahr allele (b) were purchased from Jackson Laboratories (Bar Harbor, Maine). Each strain was maintained and bred separately in the University of North Carolina at Charlotte vivarium. All animals were provided Purina Mouse Chow (Formula Number 8604 or Formula Number 2014 for pregnant and nursing females; Harlan Teklad, Indianapolis, IN) and water ad libitum. Each night, a number of females from a subset of the 6 strains were caged with males of the same strain. The following morning, each of these females was examined for the presence of a vaginal plug, which was taken as an indication of pregnancy and marked the beginning of gestation (gestation day 0; GD0).
Thirteen days after the start of gestation (GD13), each pregnant female was placed into 1 of 4 groups and dosed via oral gavage. Treatment group 1 (T1) received a dose of 0.01 μg TCDD/kg body weight, treatment group 2 (T2) received 0.1 μg TCDD/kg body weight, and treatment group 3 (T3) received 1.0 μg TCDD/kg body weight. All 3 treatment solutions were derived from an initial stock solution of TCDD (Sigma Aldrich Inc., St. Louis, MO) and corn oil that was serially diluted with additional corn oil to produce mixtures with final concentrations that allowed all groups to receive similar gavage volumes (approximately 6–11 μl). The control group (C) was given an equal volume of corn oil without TCDD. Dose selection for each mother was based on the current distribution of dosage groups within and between strains. GD13 was chosen for dosing because while the first morphological signs of tooth development are seen on GD11, the first visible signs of the M1 occur on GD13–14, and final cuspal morphology is not determined until after GD15.7
The F1 offspring of the females from each strain and treatment group were weaned and separated by sex at 28 days of age, euthanized at 70 days of age, and then skeletonized. All procedures involving the treatment of animals were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Charlotte.
Traits
Each mouse was examined for the presence or absence of both maxillary (M3) and mandibular third molars (M3) on both the left and right sides. In addition, all mice were scored as either normal or variant in mandibular M1 morphology for both molar rows. Figure 1 contains examples of both the normal (A) and variant (B–D) M1 morphologies. Normal M1s have a cleft between the first buccal and lingual cusps, while variant M1s range from simply having no cleft to having an additional cusp. A number of years ago, Sofaer8 described a similar, if not identical, trait that exhibited a (threshold) quantitative genetic basis. Altogether, 613 mice were scored for missing M3s (612 for the M1 variant), the specific sample sizes for each of the six strains being given below.
Figure 1.
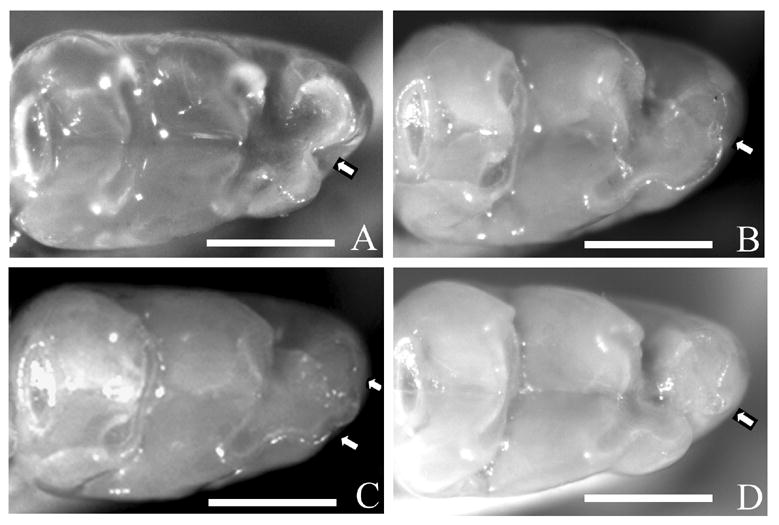
Morphological variation among first mandibular molars of C57BL/10J mice. (A) Normal morphology of right first mandibular molar. An obvious cleft is visible between the first buccal and lingual cusps (arrow). (B–D) Variant morphologies of right first mandibular molars. (B) No significant cleft is present between the first buccal and lingual cusp (arrow). (C) No significant cleft is present between the first buccal and lingual cusp, but two subtle indentations (arrows) are visible suggesting the development of an additional cusp. (D) A small additional cusp is present between the typical buccal and lingual cusps (arrow). Bars: 500 μm in A–D.
Statistical Analysis
We used logistic regression9 to test for treatment and strain effects (and their interaction) on the frequency of missing M3s and M1 variants. This was accomplished with the LOGISTIC procedure in SAS (SAS Institute Inc., Cary, NC, 2003) in which both strains and groups were entered as classification variables. Where some combinations of treatments and groups had missing data, we made use of the EXACT procedure in SAS (SAS Institute Inc., Cary, NC, 2003). In addition, we used sex as a classification variable in the logistic regression analyses.
Results
Across all treatment groups (including controls), all four M3s were present in mice of three strains (111 C57BL/6J, 112 BALB/cByJ, and 114 C57BL/10J mice), and this was nearly so for mice in the A/J strain as well (only 3 of 51 A/J mice exhibited missing third molars). However, roughly 1/3 of the mice in the CBA/J (CBA) and C3H/HeJ (C3H) strains were missing at least one M3 (see Table 1). The six strains therefore exhibited considerable heterogeneity in the proportion of third molars present or absent (on either one or both sides), this being confirmed from the results of logistic regression exact tests that showed significant strain differences for both the M3 (P = 0.0009) and the M3 (P = 0.0001).
Table 1.
The number of CBA/J and C3H/HeJ mice in each of the four dosage groups with no (0), 1 or both (2) missing third maxillary/mandibular molars.
| Strain | No. M3/M3 | 0 μg/kg (Control) | 0.01 μg/kg | 0.1 μg/kg | 1.0 μg/kg |
|---|---|---|---|---|---|
| CBA/J | 0 | 29/29 (100%/100%) | 23/21 (100%/91%) | 29/23 (100%/79%) | 0/0 (0%/0%) |
| 1 | 0/0 | 0/1 | 0/2 | 0/7 | |
| 2 | 0/0 | 0/1 | 0/4 | 30/23 | |
| Total | 29/29 | 23/23 | 29/29 | 30/30 | |
| C3H/HeJ | 0 | 23/23 (96%/96%) | 27/24 (96%/86%) | 25/24 (96%/92%) | 1/4 (3%/11%) |
| 1 | 1/1 | 1/3 | 1/2 | 5/4 | |
| 2 | 0/0 | 0/1 | 0/0 | 30/28 | |
| Total | 24/24 | 28/28 | 26/26 | 36/36 |
The percentage of mice with both third molars present also is given in parentheses.
The logistic regression tests also showed significant strain by group interactions for the presence/absence of the mandibular (P = 0.011) and maxillary third molars (P = 0.0025), so we specifically examined the proportions of missing third molars for CBA and C3H mice in each treatment group (Table 1). For both of these polymorphic strains, there are a few mice with one or more missing molars in the 0.01 and 0.1 μg/kg dosage groups, especially for the M3 in CBA mice. However, most of the missing molars occur in mice whose mothers were subjected to the highest dosage (1 μg/kg) of TCDD. Further, the association of missing mandibular and maxillary third molars in individual mice is similar over the combined CBA and C3H strains [phi (correlation) coefficient = 0.86, P < 0.01].
Using only the CBA and C3H strains, we used logistic regression to test for potential effects of strains, groups, and sex on the frequency of missing third molars. Results for strains were not statistically significant for either the mandibular or maxillary third molars, suggesting that there is no difference in the proportion of missing M3s between the CBA and C3H strains. Differences among the four treatment groups were highly significant (P < 0.01), however, so prenatal doses of TCDD appear to have affected the frequency of missing third molars. Sex reached significance (P = 0.018) for the M3, reflecting the fact that the percentage of mice missing either one or both third mandibular molars was greater in males (38%) than in females (29%). This same general trend was seen for missing M3s (32% for males, 28% for females), but was not sufficient to generate significance (P = 0.078).
The frequency of the M1 variant was low in 5 of the 6 strains, being prevalent (55%) only in the C57BL/10J mice. The variant was entirely absent in mice in the CBA and C3H strains, although it was present in a few mice in the C57BL/6J (8%), BALB/cByJ (4%) and A/J (2%) strains. An exact logistic regression test showed that the frequency of this M1 variant differed significantly (P < 0.0001) among strains. No obvious morphological variation was apparent in the maxillary molars of any of the strains.
Table 2 shows the frequency of the M1 variant in C57BL/10J mice in each of the four treatment groups. Although this variant is present in about 33% of the mice in the control group, its frequency is higher in mice in all three groups treated with TCDD, averaging about 62%. Logistic regression analysis showed that differences in the frequency of this variant among the four groups did not reach statistical significance (P = 0.078), and in addition, no sex differences were detected (P > 0.05). The percentage of variants in mice among each of three TCDD (non-control) groups does not differ (P = 0.71), however, and if these three groups are pooled, the difference in the frequency of the M1 variant between the control group and the three pooled groups is significant (P = 0.012). It therefore appears that TCDD affects the frequency of this M1 variant, although its effect is similar in mice receiving 0.01, 0.1, or 1 μg/kg doses of TCDD.
Table 2.
The number of C57BL/10 mice in each of the four dosage groups with no (0) M1s, 1 M1, or both (2) M1s showing a morphological variant.
| Strain | Number of variants | 0 μg/kg (Control) | 0.01 μg/kg | 0.1 μg/kg | 1.0 μg/kg |
|---|---|---|---|---|---|
| C57BL/10J | 0 | 18 (67%) | 9 (32%) | 11 (41%) | 13 (42%) |
| 1 | 6 | 16 | 11 | 13 | |
| 2 | 3 | 3 | 5 | 5 | |
| Total | 27 | 28 | 27 | 31 |
The percentage of mice exhibiting the normal morphology in both M1s also is given in parentheses.
Discussion
M3 Agenesis
We found that exposure to TCDD caused a significant increase in the number of missing mandibular and maxillary M3s in 2 of the 6 inbred strains evaluated (CBA and C3H). In fact, all CBA and C3H mice dosed with 1.0 μg/kg TCDD were missing at least one third mandibular molar. Our results are in agreement with other studies demonstrating that TCDD dose-dependently interferes with tooth development in vitro2 and in vivo4,10,11. For example, 1 μg/kg TCDD (but not lower doses) administered to rats on GD15 significantly decreased the proportion of M3s present in 1 of the 3 lines tested.10 However, the rat lines used by Kattainen et al.10 were chosen because they possessed different alleles at two loci associated with resistance to TCDD toxicity. Our focus was to evaluate dose responses to TCDD on molar development among a number of inbred strains, all without known resistance to TCDD.
Most of the between-strain variation in sensitivity to TCDD has been attributed to differences in its binding affinity to receptors produced by different alleles at the Ahr locus.5,6 However, differences in relative sensitivity to TCDD depend on the toxic endpoints being assessed,4,12 suggesting that genetic factors beyond those at the Ahr locus influence an organism’s response to TCDD. Our results showing M3 loss primarily in only 2 of 6 inbred strains, all of which have the susceptible (b) allele at the Ahr locus, support the idea that genes at other loci are involved in mediating the effects of TCDD on tooth development in mice.
A number of genes act to produce missing M3s in mice.13 Additionally, inter-strain differences in the prevalence of missing 3rd molars have been known for years.14 Although the frequency of missing M3s for the CBA/J strain has been reported as 3%,15 we found no missing M3s in our control CBA/J mice. However, the existence of even low frequencies of missing M3 in some inbred strains suggests that these strains have a genetically-influenced predisposition to molar agenesis that may be triggered by less than optimal prenatal conditions. Clearly the TCDD dosing in our CBA and C3H mice, at least at the 1 μg/kg level, caused a disruption sufficient to produce nearly complete M3 loss. TCDD also apparently acted on a pathway common to the development of both upper and lower molars since we found a very high correlation between the frequencies of missing maxillary and mandibular third molars. Previous studies with mutants have tended to show that the mandibular rather than the maxillary molars are more susceptible to loss.15,16,17 However, lactational exposure to TCDD has been shown to have a greater effect on M3 eruption than on M3 eruption, suggesting that, at least in the case of TCDD exposure, timing may influence the relative sensitivity of molars to developmental disruption.18
M1 Variants
Considerable variation in the degree of separation between the most anterior lingual (L1) and buccal (B1) cusps of the first mandibular molar (M1) has been reported among the CBA, C57BL, BALB/c and A inbred mouse strains.19 In particular, in the C57BL strain, these 2 cusps had very little anterior separation. In contrast, we saw a fairly prominent notch at this location in many individuals from both the C57BL/6J and C57BL/10J strains. More significantly, we observed varying degrees of abnormal lobe formation in this region, up to and including the existence of a complete additional lobe (see figure 1). As will be recalled, these variant forms were found almost exclusively in the C57BL/10J strain, occurring in over 50% of the individuals. Sofaer8 described a supernumerary cusp that was present at high frequency in this same molar region in the Tuck No. 1 strain (TUCK) and in the offspring of TUCK x C57 crosses, but not in the offspring of crosses with other inbred strains.
The frequency of the M1 variant was significantly higher in all three groups of TCDD-dosed C57BL/10J mice compared with the control. This suggests that even low doses of TCDD are sufficient to alter normal cuspal development, as has been shown to occur in vitro3 as well. It was a surprise, however, to find such a high frequency of this variant in the C57BL/10J compared to the C57BL/6J strain. These two strains differ at only three loci (h9, Igh2, and Alad), none of which appears to be directly related to tooth development (http://www.informatics.jax.org). It is possible that an unknown function of one of these genes or a mutation occurring since the strains diverged is responsible for the difference in response. More C57BL/6J mice (8) exhibited the variant M1 morphology than any strain except for the C57BL/10J strain, however, suggesting some relationship between the C57BL background and the M1 morphological variant (see also Sofaer8). The differences seen in this region of the tooth indicate it is extremely sensitive to developmental disruption and may be useful in evaluating the effects of various stressors.
The lack of an association between the frequency of missing molars and the M1 variants suggests that different pathways are affected by TCDD. This is not surprising since different genes are active at different stages of molar development and inhibition of molar formation occurs much earlier in development than crown and cusp formation.7 In contrast to our finding that upper and lower M3s respond similarly to TCDD, the maxillary M1s of the C57BL/10J did not exhibit the same (or any detectable) variant as we found in the mandibular M1s. Divergence of developmental pathways between maxillary and mandibular molars as development proceeds could explain this difference in response to TCDD. This seems reasonable since the crown size of upper and lower M1s appears to be determined primarily by different genes.20
Finally, we may well have obtained different results had we chosen a time other than GD13. We chose this time as a compromise between being sufficiently early to induce changes in the cusps yet not so early as to excessively inhibit molar formation3,10,18. We can only speculate on the changes that might have been induced by a later dosing date such as GD15, at which time the molars have advanced to the late cap stage of development7. It would be interesting in fact to test whether the M1 variant would be as frequent in C57BL/10J mice subjected to dosing with TCDD at a later date.
Acknowledgments
It is a pleasure to thank Vicki McGlosson, Norm Lefebvre, Hal Farris and the University of North Carolina vivarium staff for advice and assistance in rearing and caring for these mice, and Sue Peters, Johnny Huang and Naudine Tehrani for assistance in preparation of the mouse skeletons. Support for this research was provided by grant # 1 R21 DE 015597-01A1 from the National Institute of Dental and Craniofacial Research to L.J. Leamy.
Abbreviations
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- M1
first molar
- M1
first mandibular molar
- M3
third molar
- M3
third mandibular molar
- M3
third maxillary molar
- AHR
aryl hydrocarbon receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alaluusua S, Calderara P, Gerthoux PM, Lukinmaa P-L, Kovero O, Needham L, et al. Developmental dental aberrations after the dioxin accident in Seveso. Environmental Health Perspectives. 2004;112:1313–1318. doi: 10.1289/ehp.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Partanen A-M, Alaluusua S, Miettinen PJ, Thesleff I, Tuomisto J, Pohjanvirta R, et al. Epidermal growth factor receptor as a mediator of developmental toxicity of dioxin in mouse embryonic teeth. Laboratory Investigation. 1998;78:1473–1481. [PubMed] [Google Scholar]
- 3.Partanen A-M, Kiukkonen A, Sahlberg C, Alaluusua S, Thesleff I, Pohjanvirta R, et al. Developmental toxicity of dioxin to mouse embryonic teeth in vitro: arrest of tooth morphogenesis involves stimulation of apoptotic program in the dental epithelium. Toxicology and Applied Pharmacology. 2004;194:24–33. doi: 10.1016/j.taap.2003.08.014. includes Erratum in: Toxicl Appl Pharmacol 2005; 202:212–214. [DOI] [PubMed] [Google Scholar]
- 4.Kiukkonen A, Viluksela M, Sahlberg C, Alaluusua S, Tuomisto JT, Tuomisto J, et al. Response of the incisor tooth to 2,3,7,8-Tetrachlorodibenzo-p-dioxin in a dioxin resistant and a dioxin sensitive rat strain. Toxicological Sciences. 2002;69:482–489. doi: 10.1093/toxsci/69.2.482. [DOI] [PubMed] [Google Scholar]
- 5.Birnbaum LS. The mechanism of dioxin toxicity: relationship to risk assessment. Environmental Health Perspectives. 1994;102 (Suppl 9):157–167. doi: 10.1289/ehp.94102s9157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poland A, Palen D, Glover E. Analysis of the four alleles of the murine aryl hydrocarbon receptor. Molecular Pharmacology. 1994;46:915–921. [PubMed] [Google Scholar]
- 7.Cobourne MT, Sharpe PT. Tooth and jaw: molecular mechanisms of patterning in the first branchial arch. Archives of Oral Biology. 2003;48:1–14. doi: 10.1016/s0003-9969(02)00208-x. [DOI] [PubMed] [Google Scholar]
- 8.Sofaer JA. The genetics and expression of a dental morphological variant in the mouse. Archives of Oral Biology. 1969;14:1213–1223. doi: 10.1016/0003-9969(69)90159-9. [DOI] [PubMed] [Google Scholar]
- 9.Sokal RR, Rohlf JF. Biometry. 3. New York: W. H. Freeman; 1995. [Google Scholar]
- 10.Kattainen H, Tuukkanen J, Simanainen U, Tuomisto JT, Kovero O, Lukinmaa P-L, et al. In utero/lactational 2,3,7,8-Tetrachlorodibenzo-p-dioxin exposure impairs molar tooth development in rats. Toxicology and Applied Pharmacology. 2001;174:216–224. doi: 10.1006/taap.2001.9216. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Sahlberg C, Kiukkonen A, Alaluusua S, Pohjanvirta R, Tuomisto J, et al. Lactational exposure of Han/Wistar rats to 2,3,7,8-Tetrachlorodibenzo-p-dioxin interferes with enamel maturation and retards dentin mineralization. Journal of Dental Research. 2004;83:139–144. doi: 10.1177/154405910408300211. [DOI] [PubMed] [Google Scholar]
- 12.Poland A, Glover E. 2,3,7,8-Tetrachlorodibenzo-p-dioxin: segregation of toxicity with the Ah locus. Molecular Pharmacology. 1980;17:86–94. [PubMed] [Google Scholar]
- 13.Nomura R, Shimizu T, Asada Y, Hirukawa S, Maeda T. Genetic mapping of the absence of third molars in EL mice to chromosome 3. Journal of Dental Research. 2003;82:786–790. doi: 10.1177/154405910308201005. [DOI] [PubMed] [Google Scholar]
- 14.Grüneberg H. The genetics of tooth defect in the mouse. Proceedings of the Royal Society. 1951;138:437–451. [Google Scholar]
- 15.Murai M. A genetic study on the development of the lower molars and mandible in mice: change of the genetic and environmental effects in the course of pre- and postnatal morphogenesis. Japanese Journal of Genetics. 1975;50:73–90. [Google Scholar]
- 16.Grewal MS. The development of an inherited tooth defect in the mouse. Journal of Embryology and Experimental Morphology. 1962;10:202–211. [Google Scholar]
- 17.Miller WA. The dentitions of tabby and crinkled mice. In: Butler PM, Joysey KA, editors. Development, function and evolution of teeth. London: Academic Press; 1978. pp. 99–109. [Google Scholar]
- 18.Lukinmaa P-L, Sahlberg C, Leppaniemi A, Partanen A-M, Kovero O, Pohjanvirta R, et al. Arrest of rat molar tooth development by lactational exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin. Toxicology and Applied Pharmacology. 2001;173:38–47. doi: 10.1006/taap.2001.9155. [DOI] [PubMed] [Google Scholar]
- 19.Grüneberg H. Genes and genotypes affecting the teeth of the mouse. Journal of Embryology and Experimental Morphology. 1965;14:137–159. [PubMed] [Google Scholar]
- 20.Shimizu T, Oikawa H, Han J, Kurose E, Maeda T. Genetic analysis of crown size in the first molars using SMXA recombinant inbred mouse strains. Journal of Dental Research. 2004;83:45–49. doi: 10.1177/154405910408300109. [DOI] [PubMed] [Google Scholar]


