Abstract
Mice bearing the I-Ag7 class II major histocompatibility complex molecules contain a high number of spontaneous autoreactive T cells, as estimated by limiting-dilution assays. We found this autoreactivity in various strains that bear the I-Ag7 molecule, such as the nonobese diabetic (NOD) mouse strain, which spontaneously develops autoimmune diabetes. However, NOD mice strains that do not express the I-Ag7 molecule, but instead express I-Ab, do not have a high incidence of autoreactive T cells. About 15% of the autoreactive T cells also recognize the I-Ag7 molecule expressed in the T2 line, which is defective in the processing of protein antigens. We interpret this to mean that some of the T cells may interact with class II molecules that are either devoid of peptides or contain a limited peptide content. We also find a high component of autoreactivity among antigen-specific T cell clones. These T cell clones proliferate specifically to protein antigens but also have a high level of reactivity to antigen-presenting cells not pulsed with antigen. Thus, the library of T cell receptors in NOD mice is skewed to autoreactivity, which we speculate is based on the weak peptide-binding properties of I-Ag7 molecules.
This paper contains a functional analysis of T cells found normally in lymphoid organs of nonobese diabetic (NOD) mice. NOD mice are highly susceptible to diabetic autoimmunity and also develop other tissue pathologies (1). The major genetic trait for diabetic susceptibility in NOD is the class II major histocompatibility complex (MHC) molecule, I-Ag7 (2). I-Ag7 molecules are the equivalent of human DQ molecules, which are associated with human type I diabetes (3, 4). Many of the Caucasian type I diabetes patients carry a DQ β chain that lacks the usual Asp residue at position 57 of the β chain (3, 4). The same biochemical trait is found in the murine I-Ag7 β chain (5).
We have recently demonstrated that I-Ag7 molecules are weak peptide-binding histocompatibility molecules, regardless of the nature of the peptide (6). Peptides have weak affinity with a fast dissociation rate. Included among the peptides are the pancreatic β cell peptides that trigger diabetogenic T cells (6). In accordance with their pan-weak binding properties, I-Ag7 molecules are unstable in SDS/PAGE analysis [regardless of the source of antigen-presenting cells (APC), including those from islets; refs. 6 and 7]. The high percentage of SDS-unstable I-Ag7 molecules indicates their poor interaction with autologous and foreign peptides. Most other class II alleles bear 10–90% of molecules as SDS-stable (8, 9). Also, I-Ag7 molecules have a short half-life in APC, and peptides offered to APC of NOD have a strikingly short time of presentation to T cells (6). The I-Ag7 molecules extracted from APC have a relatively low content of peptides (10). A recent analysis of I-Ag7 molecules with a mutation of Asp at 57 to wild-type Ser disclosed that these also are weak binding molecules (6).
The weak peptide-binding characteristics of I-Ag7 prompted us to analyze whether it may be reflected on the properties of T cells found normally in lymphoid tissues. We speculated that because of the weak interactions of I-Ag7 molecules with peptides, the normal regulatory processes that result in elimination or inactivation of autoreactive T cells would not be operative at their best. The consequences will be a higher incidence of T cells reactive to a variety of self-antigens.
MATERIALS AND METHODS
Mice.
Mice were originally obtained from The Jackson Laboratory and maintained in the Washington University Animal Facility, and female mice of 4 to 8 weeks of age were used. These included: NOD, NOD.H-2b, C57BL/6 (B6), C57BL/6-H-2g7 (B6.g7), and Balb/c. B6.g7 mice do not develop diabetes. Neither do NOD.H-2b, although they show periinsulitis (11).
T Cell Experiments.
Lymphocytes were obtained from periaortic and inguinal lymph nodes of mice. Autoreactive T cells, i.e., T cells that reacted to autologous APC without the need for exogenous antigen, were estimated by limiting-dilution analysis: lymph node cells were cultured at different concentrations (from 100 to 3,000) in 200-μl wells with irradiated syngeneic spleen cells (5 × 105) and DMEM, with 10% fetal calf serum (FCS) and 50 units/ml of interleukin-2 (IL-2). Frequency of responding T cells was estimated after 10–14 days of culture (12). Antigen-specific T cells were also examined by using lymph nodes cells from mice 7 days after immunization with ovalbumin (OVA) (100 μg) in complete Freund’s adjuvant (CFA). Such T cells were stimulated in vitro and cloned also by limiting dilution using OVA. The positive cultures were then expanded further in media with 50 units/ml of IL-2, washed, and then tested with NOD or B6 spleen cells, with or without OVA. Either set of T cells (i.e., from unimmunized and OVA-immunized mice) were propagated in culture and established as clones that were then tested for their reactivity under two conditions: (i) with spleen APC for proliferation (2 × 104 T cells, 2.5 × 105, 2,000 rad irradiated spleen cells; after 3 days cells were pulsed with 1 μCi of 3H-labeled thymidine for 6 hr) and (ii) with I-Ag7 bearing APC lines, for IL-3 secretion (these were tested with 1 × 105 T cells with 1 × 105 APC, harvesting the culture media after 1 day and testing with the IL-3-dependent line FDC-3) (13). Secretion of IL-3 was a consistent and reproducible readout by using I-Ag7-transfected cell lines. These included the M12.C3 line (named C3.g7) (6) and the T2 line (T2.g7), which lacks HLA-DM (14). We also used the T2-Ak line, which expresses I-Ak (15).
RESULTS
Autoreactive T Cells in Mice Bearing H-2g7 MHC.
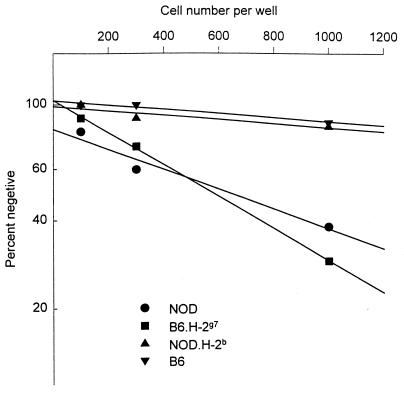
We examined the frequency of autoreactive T cells by limiting-dilution analysis. Limiting numbers of lymph node cells were stimulated with irradiated syngeneic spleen cells in microcultures in the presence of exogenous IL-2. The frequency of responding cells was calculated by linear regression and Poisson distribution analysis (12). Mice carrying the H-2g7 class II MHC molecules (NOD and B6.g7) contained higher numbers of self-reactive T cells, by about 8-fold at least, than their congenic counterparts bearing H-2b MHC (NOD.H-2b and B6) (Fig. 1 and Table 1). Immunization of NOD mice with CFA did not increase the frequency of self-reactive T cells in the draining lymph node (Table 1).
Figure 1.
Frequency of autoreactive T cells determined by limiting-dilution analysis. Limiting number of lymph node cells from indicated strain of mice (32 wells per group) were stimulated with 5 × 105 irradiated (2,000 rads) syngeneic spleen cells in the presence of 50 units/ml of IL-2 in a final volume of 200 μl 10% FCS DMEM in U-bottom microtiter plates. Cultures were scored visually under inverted microscope for the growth of the cells, and frequency of responding cells was calculated by the methods described previously (12). In this experiment frequencies of self-reactive T cells were 1 in 378 for NOD, 1 in 337 for B6.g7, and less than 1 in 3,000 for both NOD.H-2b and B6.
Table 1.
Frequency of autoreactive T cells
| Mouse strain | Frequency of autoreactive T cells |
|---|---|
| NOD (H-2g7) | 1/1,021, 1/902, 1/916, 1/1,860 |
| NOD primed with CFA† | 1/874, 1/1,112 |
| NOD.H-2b | <1/104, <1/104, <1/104 |
| B6 (H-2b) | <1/104, <1/5 × 104 |
| B6.g7 | 1/862, 1/1,342, 1/1,445 |
Frequency of self-reactive T cells was determined by the same methods described in Fig. 1. Each determination represents a different experiment. (Data shown in Fig. 1 is not included here.)
NOD mice were immunized with CFA (100 μl of 1:1 mixture of CFA and PBS) at base of the tail. Seven days after immunization, frequency of autoreactive T cells in draining lymph nodes was determined.
The specificity of the autoreactive T cells was studied further by examining T cell clones. T cell clones were established by weekly restimulation of lymph node cells with irradiated spleen cells. After three cycles of in vitro stimulation, the T cells were cloned under limiting dilution conditions (at one cell per well) in the presence of irradiated spleen cells and IL-2. Cells from positive wells were expanded further and then tested for the reactivity to the spleen stimulator cells from NOD, Balb/c, and B6 mice.
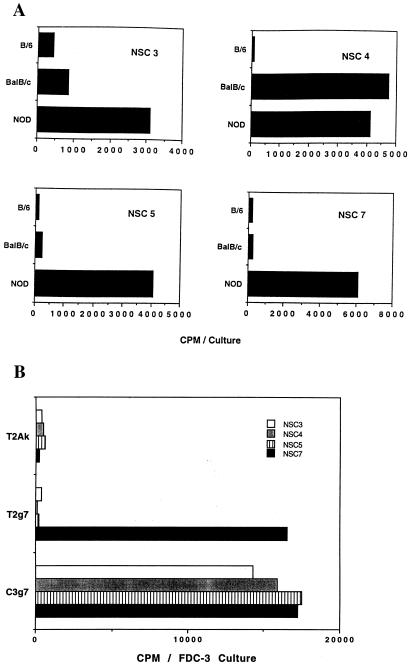
As expected, all of the clones responded to NOD spleen cells but not to B6 spleen stimulator cells. All of T cell clones established were of CD4+, CD8− phenotype, which was evident by surface immunofluorescence (data not shown). Results with four representative clones are shown in Fig. 2A. Note that one clone, NSC-4, showed cross-reactivity to Balb/c spleen cells. Of a total of 13 clones examined, only 1, NSC-4, had reactivity to Balb/c APC.
Figure 2.
Reactivity of NOD-derived autoreactive T cells. Autoreactive T cell clones were established by stimulating lymph node cells with syngeneic spleen cells and limiting dilution cloning (1 cell per well). (A) Established T cell clones (NSC 3, 4, 5, and 7) (2 × 104) were stimulated with 2.5 × 105 irradiated spleen cells. After 3 days of incubation and a 6-hr pulse with 3H-labeled thymidine, cultures were harvested for counting. (B) The same T cell clones (1 × 105) were stimulated with indicated B lymphoma cell line (1 × 105). After 24 hr of incubation, culture supernatants were harvested and tested for IL-3 activity by using the IL-3-dependent cell line FDC-3. (□, NSC3; ░⃞, NSC4; ▥, NSC5; and ▪, NSC7).
To examine further the nature of T cell reactivity to self class II molecules we tested the clones against the T2 line expressing I-Ag7. This cell line is defective in HLA-DM genes and is defective in processing protein antigens to bind to class II MHC molecules (14). Self-reactive T cell clones were stimulated by I-Ag7 expressed in the conventional B cell line C3.g7 and also in the T2 lines T2.g7 and T2-Ak for 24 hr, and IL-3 activity in the culture supernatants was measured. Fig. 2B shows the results with the same four clones. Note that the NSC-7 clone reacts with T2.g7. None reacted with T2 line bearing I-Ak. In three different experiments testing for autoreactive clones, all 47 reacted with C3.g7 APC, and 7 of the 47 clones also showed reactivity to T2.g7 cells. Thus, a significant fraction of self-reactive T cell clones (about 15%) recognize I-Ag7 class II MHC molecule lacking peptides derived from conventional self-protein antigens.
A Component of Self-Reactivity in Antigen-Specific T Cell Clones.
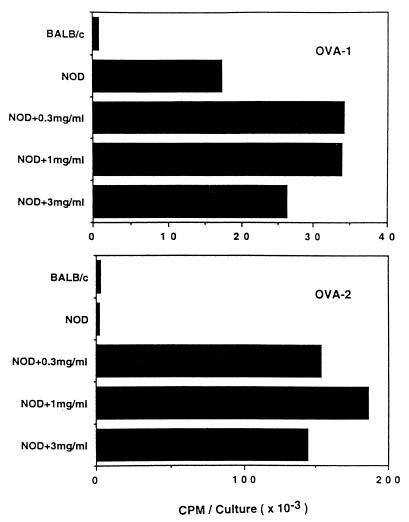
In our studies with antigen-specific T cell clones derived from NOD mice, we have frequently noted a component of autoreactivity, a high background proliferation to APC not pulsed with the antigenic peptide. To examine this issue further, we generated a number of short-term OVA-specific T cell clones from immunized NOD and B6 mice. The T cell clones were established from inguinal lymph nodes of mice 7 days after immunization with OVA in CFA. These clones were tested for their autoreactivity. In Table 2, the reactivity to OVA-pulsed APC and to APC alone were examined in T cell clones derived from either NOD or B6 mice. Among 14 NOD-derived OVA-specific clones tested, 4 clones exhibited significant reactivity to self APC, whereas no B6-derived clone showed reactivity to APC alone (Table 2). Further examination of two representative NOD clones is shown in Fig. 3. The OVA-2 clone was OVA-specific without a component of self-reactivity, as evident by the low proliferation to APC of NOD in the absence of OVA. In contrast, the OVA-1 clone reacted to NOD APC in the absence of OVA, but its reactivity was enhanced by addition of the specific antigen OVA.
Table 2.
Autoreactivity of short-term OVA-reactive T cell clones*
| Clones derived from | Stimulation
with
|
|||
|---|---|---|---|---|
| NOD + Ag | NOD | B6 + Ag | B6 | |
| NOD | ||||
| 1 | 8.9† | 0.3 | 0.1 | 0.2 |
| 2 | 25.6 | 0.2 | 0.2 | 0.7 |
| 3 | 35.1 | 9.5 | 0.3 | 0.6 |
| 4 | 53.7 | 0.6 | 0.2 | 0.3 |
| 5 | 7.6 | 0.7 | 0.6 | 0.6 |
| 6 | 52.3 | 0.6 | 0.2 | 0.7 |
| 7 | 56.6 | 1.0 | 0.8 | 0.5 |
| 8 | 9.2 | 0.3 | 0.1 | 0.8 |
| 9 | 18.6 | 1.2 | 0.1 | 0.1 |
| 10 | 16.8 | 1.1 | 4.1 | 3.2 |
| 11 | 34.9 | 26.8 | 7.3 | 5.2 |
| 12 | 13.4 | 9.8 | 1.1 | 2.4 |
| 13 | 7.6 | 0.1 | 0.1 | 0.1 |
| 14 | 1.7 | 0.1 | 0.2 | 0.2 |
| B6 | ||||
| 1 | 0.2 | 0.3 | 8.3 | 0.1 |
| 2 | 0.2 | 0.3 | 15.4 | 0.2 |
| 3 | 0.1 | 0.2 | 65.3 | 0.4 |
| 4 | 0.3 | 0.2 | 99.1 | 0.3 |
| 5 | 0.4 | 0.2 | 8.2 | 0.1 |
| 6 | 0.2 | 0.2 | 6.1 | 0.2 |
| 7 | 0.2 | 0.2 | 9.4 | 0.2 |
| 8 | 0.2 | 0.1 | 7.9 | 0.1 |
| 9 | 0.1 | 0.1 | 7.0 | 0.1 |
| 10 | 0.3 | 0.1 | 5.8 | 0.2 |
| 11 | 0.2 | 0.1 | 3.1 | 0.1 |
| 12 | 1.4 | 0.5 | 42.9 | 0.5 |
| 13 | 0.1 | 0.1 | 3.6 | 0.1 |
| 14 | 0.2 | 0.2 | 15.7 | 0.1 |
OVA-specific T cell clones were established by culturing lymph node cells from OVA immunized mice and cloning under limiting-dilution condition. Cells in the positive wells were washed, split into four wells, and stimulated with irradiated spleen cells (2.5 × 105 per well) in the presence or absence of OVA (0.5 mg/ml). Cultures were harvested after 3 days of incubation and a 6-hr pulse with 3H-labeled thymidine.
CPM/culture (× 10−3).
Figure 3.
Autoreactivity of OVA-reactive T cell clone. OVA-specific T cell clones were established by the same methods as described in Table 2. Established T cell clones (OVA-1 and 2) (2 × 104) were stimulated with 2.5 × 105 spleen cells alone or in the presence of an indicated dose of OVA. After 3 days of culture and a 6-hr pulse with 3H-labeled thymidine, cultures were harvested for counting.
DISCUSSION
In essence, the two main results shown here are, first, the high frequency of autoreactive T cells found in the two strains bearing I-Ag7 and, second, the presence of a component of self-reactivity in about one-third of antigen-specific T cell lines generated from NOD mice. These results have to be analyzed in the context of a third result in the recent study by Ridgway et al. (16). In their case, immunization of NOD mice with self proteins in Freund’s adjuvant resulted in high proliferation of lymph node cells without the need of antigen challenge. Our two results, although not identical, are in fact complimentary. The point that we make is that one does not need to immunize to detect the spontaneous autoreactive T cells. As shown here, we sampled the entire population of potential autoreactive T cells by using IL-2 under limiting-dilution conditions.
These results likely are the consequence of I-Ag7 molecules being a weak peptide-binding molecule: the expectation is that with the fast dissociation rates of peptides and a low overall content of peptides, thymic-selective mechanisms would not operate at their full potential. Thus, negative selection would not be at its optimum in purging the repertoire of self-reactive T cells. Note that about 15% autoreactive T cells reacted with I-Ag7 from the T2 line lacking HLA-DM. We interpret our results to indicate that some of the autoreactive T cells recognize I-Ag7 lacking the full repertoire of autologous peptides. Without its accessory function, the I-Ag7 in the T2 line is expected to be lacking autologous peptides and to be mostly occupied, if at all, by limited species of them, like the CLIP peptide (17). Our interpretation of this finding is that NOD biases its T cell receptor repertoire to recognition of empty I-Ag7 molecules.
The presence of a self-reactive component in about one-third of OVA peptide-specific T cells represents a second peculiar finding in NOD mice, much along the lines of the previous discussion. Again, this component could be explained by selection of T cell with receptors in which the peptide-specific determinant does not represent a dominant component. However, we have not ruled out that NOD T cells may have a higher frequency of more than one α chain expression to explain the diversity of such T cells (18). Regardless, this finding of dual specificity, i.e., autoreactive plus OVA-peptide reactive, could have biological significance: in the framework of a weak peptide interaction by APC, the second autoreactive component could contribute to the expansion and activation of such T cells.
The larger repertoire of self-reactive T cell clones in NOD mice would favor the different manifestations of T cell autoimmunity. It is noteworthy, however, that the presence of the I-Ag7 molecule is not the sole requirement for autoimmunity: it is well recognized that autoimmunity requires the concomitant expression of more than one gene product (11). Regardless, NOD autoimmunity operates under different sets of circumstances from conventional T cell reactivities in that, as shown herein and in previous studies, it is under the framework of low-affinity MHC–peptide interaction. The only situation akin to NOD autoimmunity is that found in mice bearing I-Au immunized with an encephalitogenic epitope. The peptide has a very weak binding interaction, but nevertheless induces autoimmune encephalomyelitis (19). All together, these findings raise provocative questions regarding NOD autoreactive T cells and form the basis for our future studies.
Acknowledgments
We thank Dr. P. Cresswell for the T2 and T2.Ak cell lines and Dr. J. Katz for comments. This work was supported by National Institutes of Health and Juvenile Diabetes Foundation grants.
ABBREVIATIONS
- NOD
nonobese diabetic
- MHC
major histocompatibility complex
- APC
antigen-presenting cells
- IL-2
interleukin-2
References
- 1.Kikutani H, Makino S. Adv Immunol. 1992;51:285–322. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- 2.Hattori M, Buse J B, Jackson R A, Glimcher L, Dorf M E, et al. Science. 1986;231:733–736. doi: 10.1126/science.3003909. [DOI] [PubMed] [Google Scholar]
- 3.Todd J A, Bell J I, McDevitt H O. Nature (London) 1987;329:599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 4.Morel P A, Dorman J S, Todd J A, McDevitt H O, Trucco M. Proc Natl Acad Sci USA. 1988;85:8111–8115. doi: 10.1073/pnas.85.21.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acha O H, McDevitt H O. Proc Natl Acad Sci USA. 1987;84:2435–2439. doi: 10.1073/pnas.84.8.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrasco M E, Shimizu J, Kanagawa O, Unanue E R. J Immunol. 1996;156:450–457. [PubMed] [Google Scholar]
- 7.Reizis B, Eisenstein M, Bockova J, Konen W S, Mor F, Elias D, Cohen I R. Intl Immunol. 1997;9:43–48. doi: 10.1093/intimm/9.1.43. [DOI] [PubMed] [Google Scholar]
- 8.Germain R N, Hendrix L R. Nature (London) 1991;353:134–136. doi: 10.1038/353134a0. [DOI] [PubMed] [Google Scholar]
- 9.Nelson C A, Petzold S J, Unanue E R. Proc Natl Acad Sci USA. 1993;90:1227–1231. doi: 10.1073/pnas.90.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reich E P, von Grafenstein H, Barlow A, Swenson K E, Williams K, Janeway C J. J Immunol. 1994;152:2279–2288. [PubMed] [Google Scholar]
- 11.Wicker L S, Todd J A, Peterson L B. Annu Rev Immunol. 1995;13:179–184. doi: 10.1146/annurev.iy.13.040195.001143. [DOI] [PubMed] [Google Scholar]
- 12.Kanagawa O, Louis J, Cerottini J C. J Immunol. 1982;128:2362–2369. [PubMed] [Google Scholar]
- 13.Kelso A. J Immunol. 1986;136:2930–2938. [PubMed] [Google Scholar]
- 14.Denzin L K, Robbins N F, Carboy N C, Cresswell P. Immunity. 1994;1:595–602. doi: 10.1016/1074-7613(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 15.Albert L J, Ghumman B, Watts T H. J Immunol. 1996;157:2247–2254. [PubMed] [Google Scholar]
- 16.Ridgway W M, Fasso M, Lanctot A, Garvey C, Fathman C G. J Exp Med. 1996;183:1657–1663. doi: 10.1084/jem.183.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riberdy J M, Newcomb J R, Surman M J, Barbosa J A, Cresswell P. Nature (London) 1992;360:474–477. doi: 10.1038/360474a0. [DOI] [PubMed] [Google Scholar]
- 18.Zal T, Weiss S, Mellor A, Stockinger B. Proc Natl Acad Sci USA. 1996;93:9102–9107. doi: 10.1073/pnas.93.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fairchild P J, Wraith D C. Immunol Today. 1996;17:80–85. doi: 10.1016/0167-5699(96)80584-6. [DOI] [PubMed] [Google Scholar]