Abstract
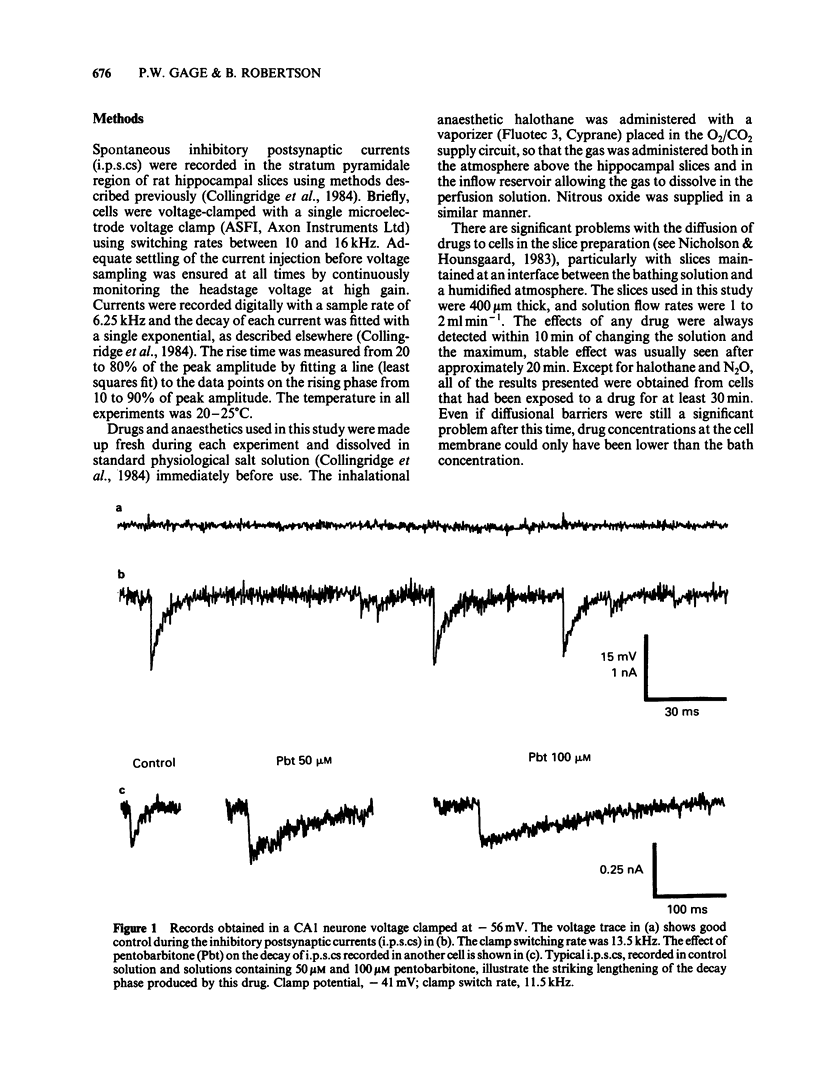
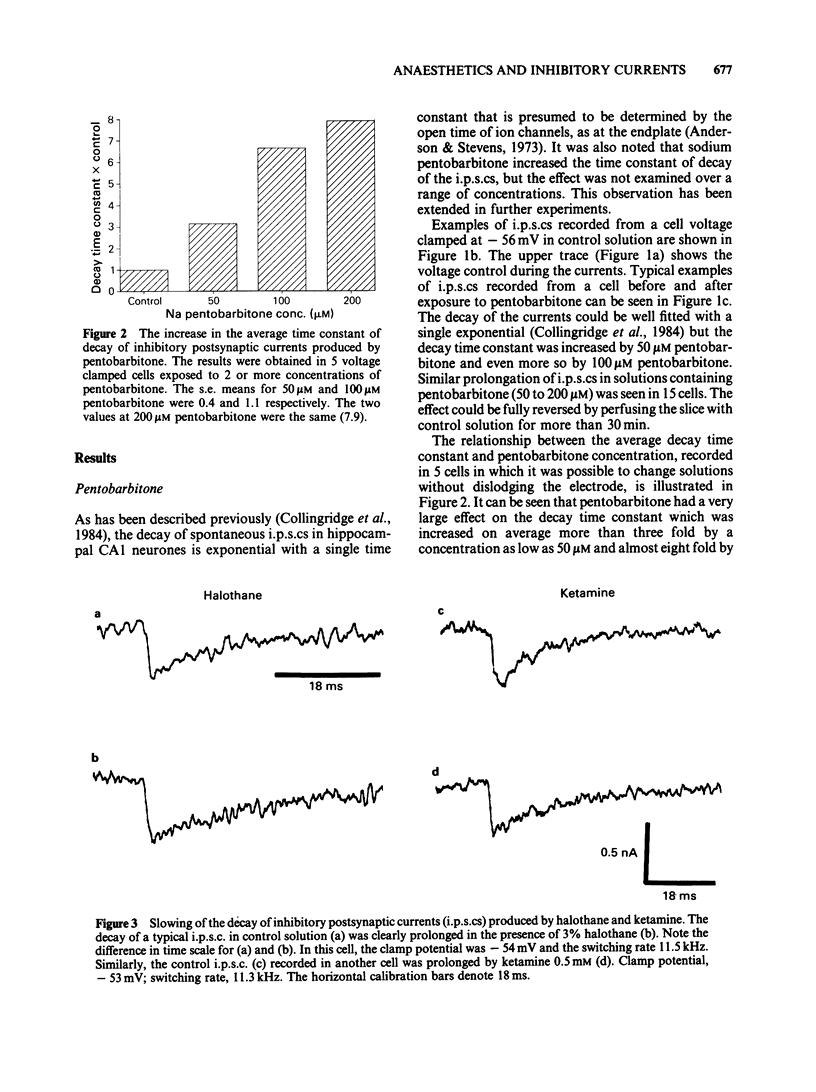
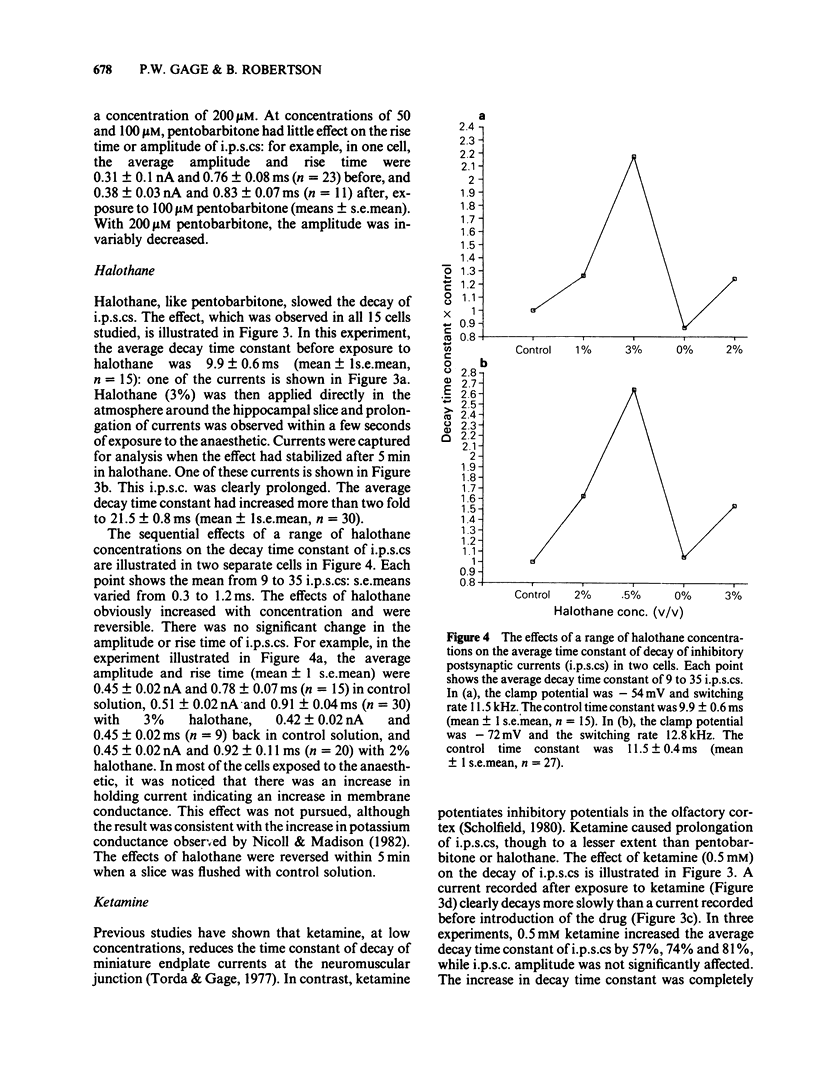
Spontaneous inhibitory postsynaptic currents (i.p.s.cs) were recorded in voltage-clamped CA1 neurones in rat hippocampal slices. The exponential decay of i.p.s.cs was prolonged by concentrations of sodium pentobarbitone as low as 50 microM. With concentrations up to 100 microM, there was no change in the amplitude or rise time of the currents but current amplitude was depressed at 200 microM. The prolongation of currents increased with drug concentration within the range tested (50 to 200 microM). Halothane, at concentrations from 1 to 5%, also increased the time constant of decay of i.p.s.cs. The effect increased with concentration and was fully reversible. Ketamine, at a concentration of 0.5 mM, increased the time constant of decay of i.p.s.cs by 50 to 80% and the effect was reversible. Ethanol (10-200 mM), nitrous oxide (75-80%), and caffeine (10 microM-5 mM) had no detectable effect on the i.p.s.cs. It is suggested that pentobarbitone, halothane and ketamine increase the time constant of decay of the i.p.s.cs by stabilizing the open state of channels activated by gamma-aminobutyric acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Gage P. W., Hamill O. P. Ethanol reduces excitatory postsynaptic current duration at a crustacean neuromuscular junction. Nature. 1977 Apr 21;266(5604):739–741. doi: 10.1038/266739a0. [DOI] [PubMed] [Google Scholar]
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Deisz R. A. Pentobarbitone interference with inhibitory synaptic transmission in crayfish stretch receptor neurones. J Physiol. 1981 Jun;315:175–187. doi: 10.1113/jphysiol.1981.sp013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C., Hulands G. H., Nunn J. F., Kitching J. A., Macdonald A. C. The effect of inhalational anaesthetics on the microtubular system in Actinosphaerium nucleofilum. J Cell Sci. 1970 Sep;7(2):483–499. doi: 10.1242/jcs.7.2.483. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anis N. A., Berry S. C., Burton N. R., Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983 Jun;79(2):565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen P. L., Gurevich N., Durand D. Ethanol in low doses augments calcium-mediated mechanisms measured intracellularly in hippocampal neurons. Science. 1982 Jan 15;215(4530):306–309. doi: 10.1126/science.7053581. [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Chan S. L., Way W. L., Trevor A. J. Distribution in the brain and metabolism of ketamine in the rat after intravenous administration. Anesthesiology. 1973 Oct;39(4):370–376. doi: 10.1097/00000542-197310000-00003. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Gage P. W., Robertson B. Inhibitory post-synaptic currents in rat hippocampal CA1 neurones. J Physiol. 1984 Nov;356:551–564. doi: 10.1113/jphysiol.1984.sp015482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger W., Stettmeier H. Postsynaptic actions of ethanol and methanol in crayfish neuromuscular junctions. Pflugers Arch. 1984 Feb;400(2):113–120. doi: 10.1007/BF00585028. [DOI] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N., Schneider G. T. Effects of some aliphatic alcohols on the conductance change caused by a quantum of acetylcholine at the toad end-plate. J Physiol. 1975 Jan;244(2):409–429. doi: 10.1113/jphysiol.1975.sp010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo A. Effects of procaine, pentobarbital and halothane on synaptic transmission in the central nervous system. J Pharmacol Exp Ther. 1969 Oct;169(2):185–195. [PubMed] [Google Scholar]
- Gruol D. L. Ethanol alters synaptic activity in cultured spinal cord neurons. Brain Res. 1982 Jul 8;243(1):25–33. doi: 10.1016/0006-8993(82)91117-9. [DOI] [PubMed] [Google Scholar]
- Hinkley R. E., Telser A. G. The effects of halothane on cultured mouse neuroblastoma cells. I. Inhibition of morphological differentiation. J Cell Biol. 1974 Nov;63(2 Pt 1):531–540. doi: 10.1083/jcb.63.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Richards C. D. Barbiturate potentiation of hippocampal i.p.s.p.s is not mediated by blockade of GABA uptake [proceedings]. J Physiol. 1977 Jul;269(1):42P–44P. [PubMed] [Google Scholar]
- LARRABEE M. G., POSTERNAK J. M. Selective action of anesthetics on synapses and axons in mammalian sympathetic ganglia. J Neurophysiol. 1952 Mar;15(2):91–114. doi: 10.1152/jn.1952.15.2.91. [DOI] [PubMed] [Google Scholar]
- Mathers D. A., Barker J. L. Chemically induced ion channels in nerve cell membranes. Int Rev Neurobiol. 1982;23:1–34. doi: 10.1016/s0074-7742(08)60620-0. [DOI] [PubMed] [Google Scholar]
- Nestoros J. N. Ethanol specifically potentiates GABA-mediated neurotransmission in feline cerebral cortex. Science. 1980 Aug 8;209(4457):708–710. doi: 10.1126/science.7394531. [DOI] [PubMed] [Google Scholar]
- Newlin S. A., Mancillas-Trevino J., Bloom F. E. Ethanol causes increases in excitation and inhibition in area CA3 of the dorsal hippocampus. Brain Res. 1981 Mar 23;209(1):113–128. doi: 10.1016/0006-8993(81)91175-6. [DOI] [PubMed] [Google Scholar]
- Nicholson C., Hounsgaard J. Diffusion in the slice microenvironment and implications for physiological studies. Fed Proc. 1983 Sep;42(12):2865–2868. [PubMed] [Google Scholar]
- Nicoll R. A., Eccles J. C., Oshima T., Rubia F. Prolongation of hippocampal inhibitory postsynaptic potentials by barbiturates. Nature. 1975 Dec 18;258(5536):625–627. doi: 10.1038/258625a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Madison D. V. General anesthetics hyperpolarize neurons in the vertebrate central nervous system. Science. 1982 Sep 10;217(4564):1055–1057. doi: 10.1126/science.7112112. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The effects of anaesthetics on synaptic excitation and inhibition in the olfactory bulb. J Physiol. 1972 Jun;223(3):803–814. doi: 10.1113/jphysiol.1972.sp009875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistri A., Berti C. Caffeine-induced potentiation of GABA effects on frog spinal cord: an electrophysiological study. Brain Res. 1983 Jan 10;258(2):263–270. doi: 10.1016/0006-8993(83)91149-6. [DOI] [PubMed] [Google Scholar]
- Nistri A., Constanti A. Pharmacological characterization of different types of GABA and glutamate receptors in vertebrates and invertebrates. Prog Neurobiol. 1979;13(2):117–235. doi: 10.1016/0301-0082(79)90016-9. [DOI] [PubMed] [Google Scholar]
- Scholfield C. N. Potentiation of inhibition by general anaesthetics in neurones of the olfactory cortex in vitro. Pflugers Arch. 1980 Feb;383(3):249–255. doi: 10.1007/BF00587527. [DOI] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: voltage-clamp analysis of inhibitory synaptic connections. J Neurophysiol. 1984 Sep;52(3):469–487. doi: 10.1152/jn.1984.52.3.469. [DOI] [PubMed] [Google Scholar]
- Torda T. A., Gage P. W. Postsynaptic effect of i.v. anaesthetic agents at the neuromuscular junction. Br J Anaesth. 1977 Aug;49(8):771–776. doi: 10.1093/bja/49.8.771. [DOI] [PubMed] [Google Scholar]
- Willow M., Johnston G. A. Enhancement of GABA binding by pentobarbitone. Neurosci Lett. 1980 Jul;18(3):323–327. doi: 10.1016/0304-3940(80)90305-5. [DOI] [PubMed] [Google Scholar]


