Abstract
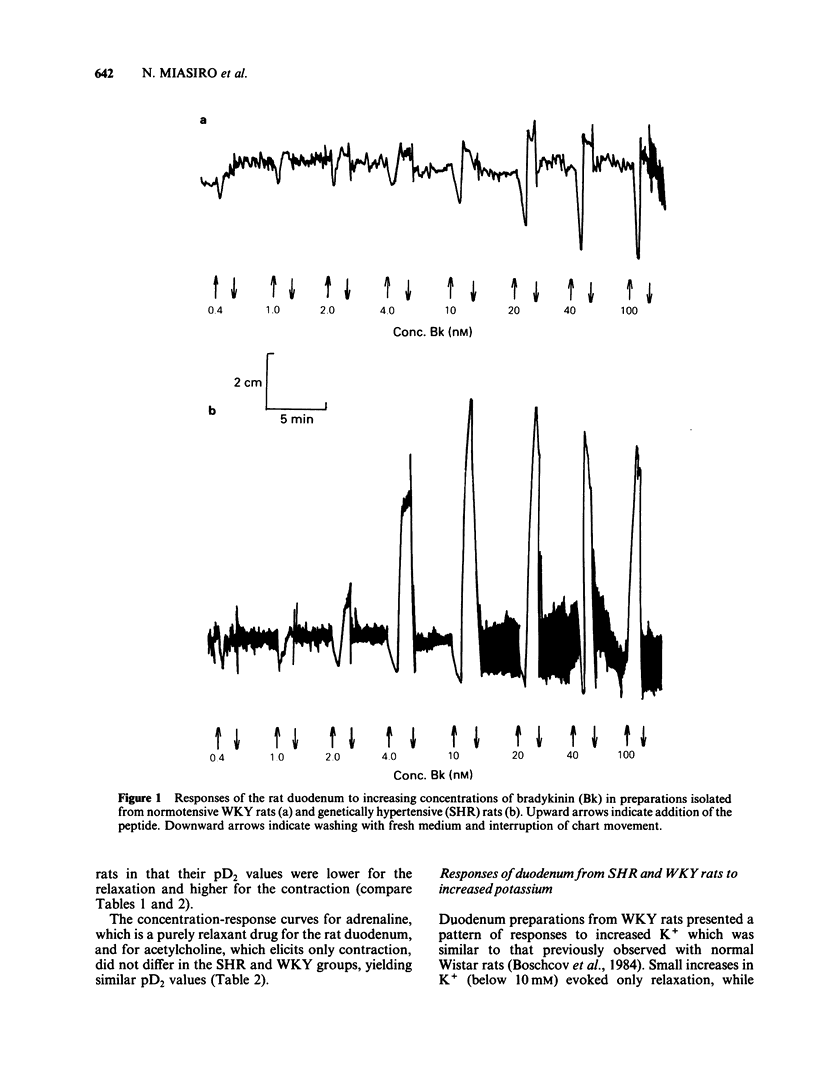
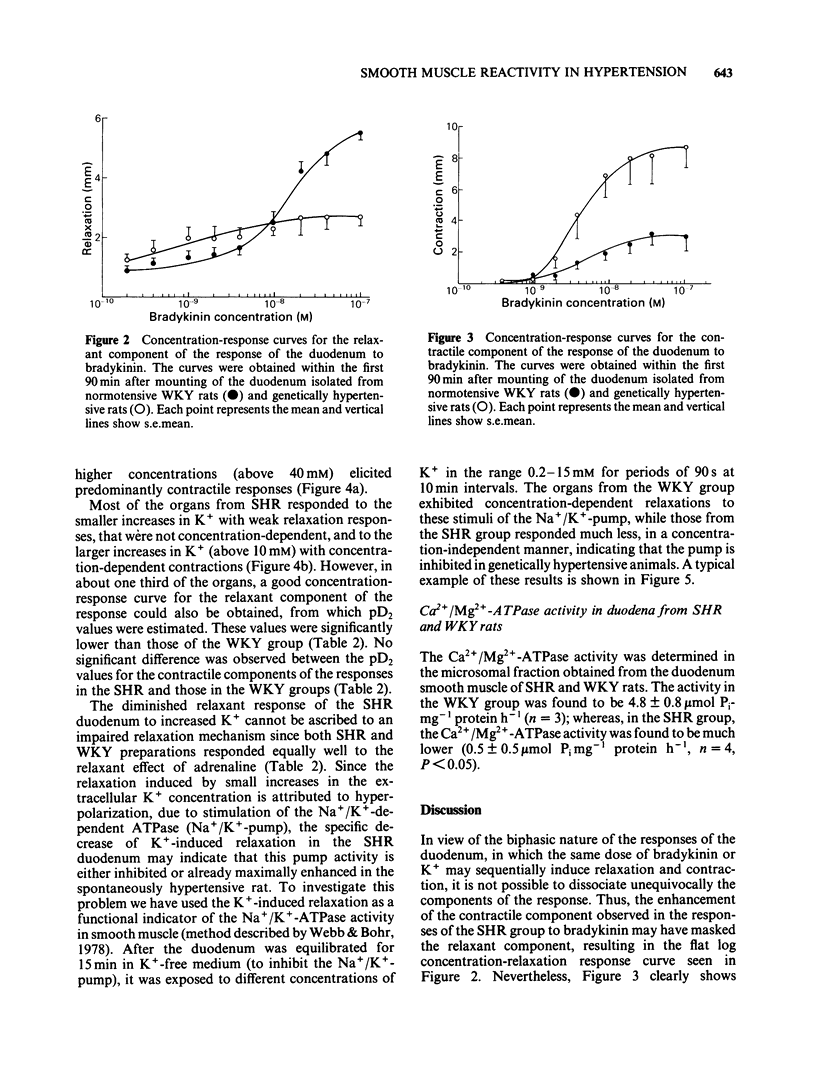
The biphasic (relaxation-contraction) response of the isolated duodenum was used to study the reactivity of non-vascular smooth muscles in genetic (SHR) and renal hypertensive rats compared to their respective controls (WKY and Wistar). For the contractile component of the response to bradykinin, the duodenum from WKY rats was more sensitive, whereas the duodenum from SHR was both more sensitive and hyperreactive, compared to that from Wistar rats. The relaxant component of the response to bradykinin was present in the duodenum of both WKY rats and SHR, but was concentration-dependent only in the WKY group. The relaxant response to K+ was very small in SHR, and was not concentration-dependent. The concentration-response curves for relaxant responses to adrenaline and for contractile responses to acetylcholine did not differ in the SHR and WKY groups. Ca2+/Mg2+-ATPase activity was found to be markedly reduced in the SHR group. No qualitative or quantitative differences were observed between the responses of the duodenum of renal hypertensive rats and those of their normotensive controls. It is proposed that the altered reactivity of the SHR duodenum is due to changes in ion handling by the smooth muscle cell membrane.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J., Da Ponte F., Worcel M. Evidence for a visceral smooth muscle abnormality in Okamoto spontaneous hypertension. Br J Pharmacol. 1977 Apr;59(4):621–625. doi: 10.1111/j.1476-5381.1977.tb07730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio A. The relaxing effect of bradykinin on intestinal smooth muscle. Br J Pharmacol Chemother. 1968 Jan;32(1):78–86. doi: 10.1111/j.1476-5381.1968.tb00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschcov P., Paiva A. C., Paiva T. B., Shimuta S. I. Further evidence for the existence of two receptor sites for bradykinin responsible for the diphasic effect in the rat isolated duodenum. Br J Pharmacol. 1984 Oct;83(2):591–600. doi: 10.1111/j.1476-5381.1984.tb16523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A., Bülbring E., Tomita T. The effect of sodium and calcium on the action potential of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1969 Feb;200(3):637–654. doi: 10.1113/jphysiol.1969.sp008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield M. P., Paterson G., Wayyes A. R. Altered responses to noradrenaline of vasa deferentia from genetically hypertensive rats [proceedings]. J Physiol. 1977 Mar;266(1):18P–19P. [PubMed] [Google Scholar]
- Corbett D. A., Goldberg M. T., Swamy V. C., Triggle C. R., Triggle D. J. Reactivity of vasa deferentia from spontaneously hypertensive and normotensive Wistar rats. Can J Physiol Pharmacol. 1980 Jun;58(6):656–665. doi: 10.1139/y80-108. [DOI] [PubMed] [Google Scholar]
- Faber D. B., Van der Meer C. A study of some bradykinin potentiating peptides derived from plasma proteins. Arch Int Pharmacodyn Ther. 1973 Oct;205(2):226–243. [PubMed] [Google Scholar]
- KURIYAMA H. The influence of potassium, sodium and chloride on the membrane potential of the smooth muscle of taenia coli. J Physiol. 1963 Apr;166:15–28. doi: 10.1113/jphysiol.1963.sp007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan C. Y., Belbeck L., Daniel E. E. Abnormal biochemistry of vascular smooth muscle plasma membrane as an important factor in the initiation and maintenance of hypertension in rats. Blood Vessels. 1979;16(5):259–268. doi: 10.1159/000158214. [DOI] [PubMed] [Google Scholar]
- Kwan C. Y., Belbeck L., Daniel E. E. Abnormal biochemistry of vascular smooth muscle plasma membrane isolated from hypertensive rats. Mol Pharmacol. 1980 Jan;17(1):137–140. [PubMed] [Google Scholar]
- Kwan C. Y., Grover A. K., Sakai Y. Abnormal biochemistry of subcellular membranes isolated from nonvascular smooth muscles of spontaneously hypertensive rats. Blood Vessels. 1982;19(6):273–283. doi: 10.1159/000158394. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moore L., Hurwitz L., Davenport G. R., Landon E. J. Energy-dependent calcium uptake activity of microsomes from the aorta of normal and hypertensive rats. Biochim Biophys Acta. 1975 Dec 16;413(3):432–443. doi: 10.1016/0005-2736(75)90126-1. [DOI] [PubMed] [Google Scholar]
- Overbeck H. W., Grissette D. E. Sodium pump activity in arteries of rats with Goldblatt hypertension. Hypertension. 1982 Jan-Feb;4(1):132–139. doi: 10.1161/01.hyp.4.1.132. [DOI] [PubMed] [Google Scholar]
- Postnov Y. V., Orlov S. N., Pokudin N. I. Decrease of calcium binding by the red blood cell membrane in spontaneously hypertensive rats and in essential hypertension. Pflugers Arch. 1979 Mar 16;379(2):191–195. doi: 10.1007/BF00586947. [DOI] [PubMed] [Google Scholar]
- SCHAFFENBURG C. A. Device to control constriction of main renal artery for production of hypertension in small animals. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:676–677. doi: 10.3181/00379727-101-25058. [DOI] [PubMed] [Google Scholar]
- Sabia E. B., Tominaga M., Paiva A. C., Paiva T. B. Bradykinin potentiating and sensitizing activities of new synthetic analogues of snake venom peptides. J Med Chem. 1977 Dec;20(12):1679–1681. doi: 10.1021/jm00222a030. [DOI] [PubMed] [Google Scholar]
- Sowers J. R., Beck F., Stern N., Raghavan S. R. Reduced sodium-potassium dependent ATPase and its possible role in the development of hypertension in spontaneously hypertensive rats. Clin Exp Hypertens A. 1983;5(1):71–86. doi: 10.3109/10641968309048811. [DOI] [PubMed] [Google Scholar]
- Webb R. C., Bohr D. F. Potassium relaxation of vascular smooth muscle from spontaneously hypertensive rats. Blood Vessels. 1979;16(2):71–79. [PubMed] [Google Scholar]
- Webb R. C., Bohr D. F. Potassium-induced relaxation as an indicator of Na+-K+ ATPase activity in vascular smooth muscle. Blood Vessels. 1978;15(1-3):198–207. doi: 10.1159/000158166. [DOI] [PubMed] [Google Scholar]
- Wei J. W., Janis R. A., Daniel E. E. Studies on subcellular fractions from mesenteric arteries of spontaneously hypertensive rats: alterations in both calcium uptake and enzyme activities. Blood Vessels. 1976;13(5):293–308. doi: 10.1159/000158099. [DOI] [PubMed] [Google Scholar]
- Winquist R. J., Webb R. C., Bohr D. F. Vascular smooth muscle in hypertension. Fed Proc. 1982 Jun;41(8):2387–2393. [PubMed] [Google Scholar]
- Yanagawa T., Yamamoto N., Suzuki A. The modification of the responses to noradrenaline and acetylcholine by aging and the effects of some smooth muscle relaxants on the smooth muscle contractile drugs. Experiment in the isolated vas deferens of SHRSP and Wistar-Kyoto rats. Jpn Heart J. 1978 Jul;19(4):642–643. doi: 10.1536/ihj.19.642. [DOI] [PubMed] [Google Scholar]


