Abstract
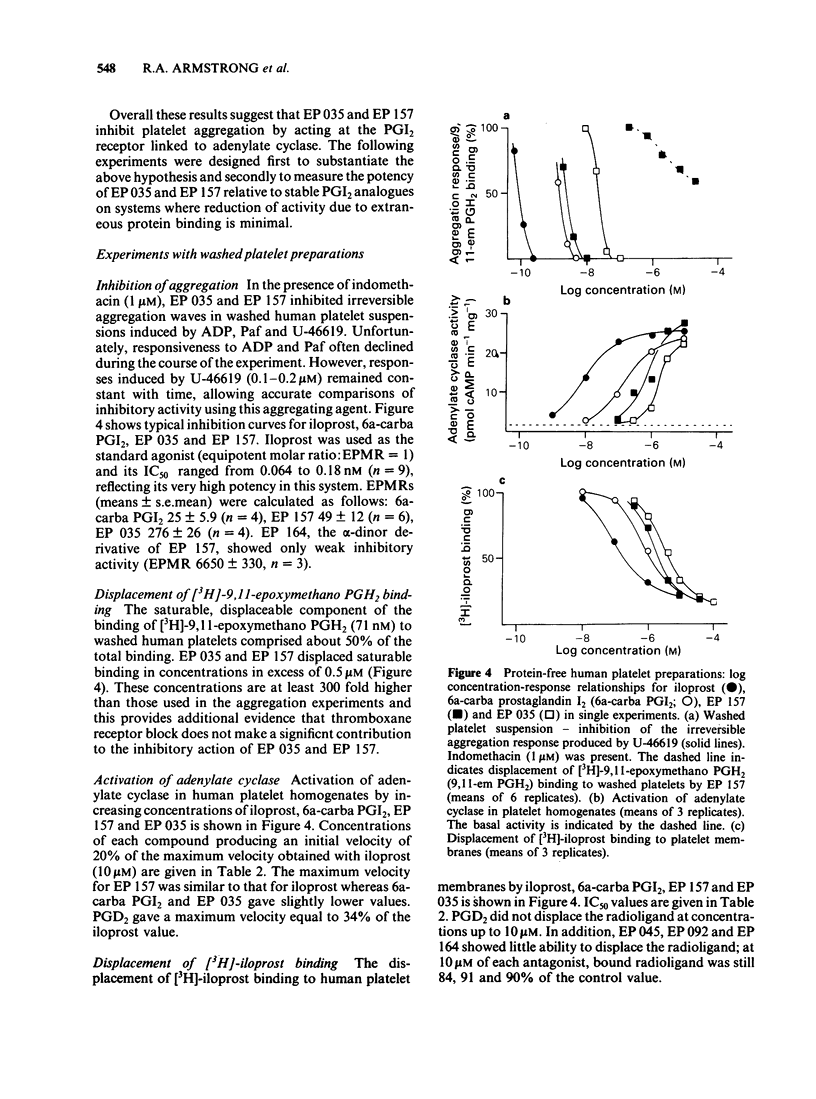
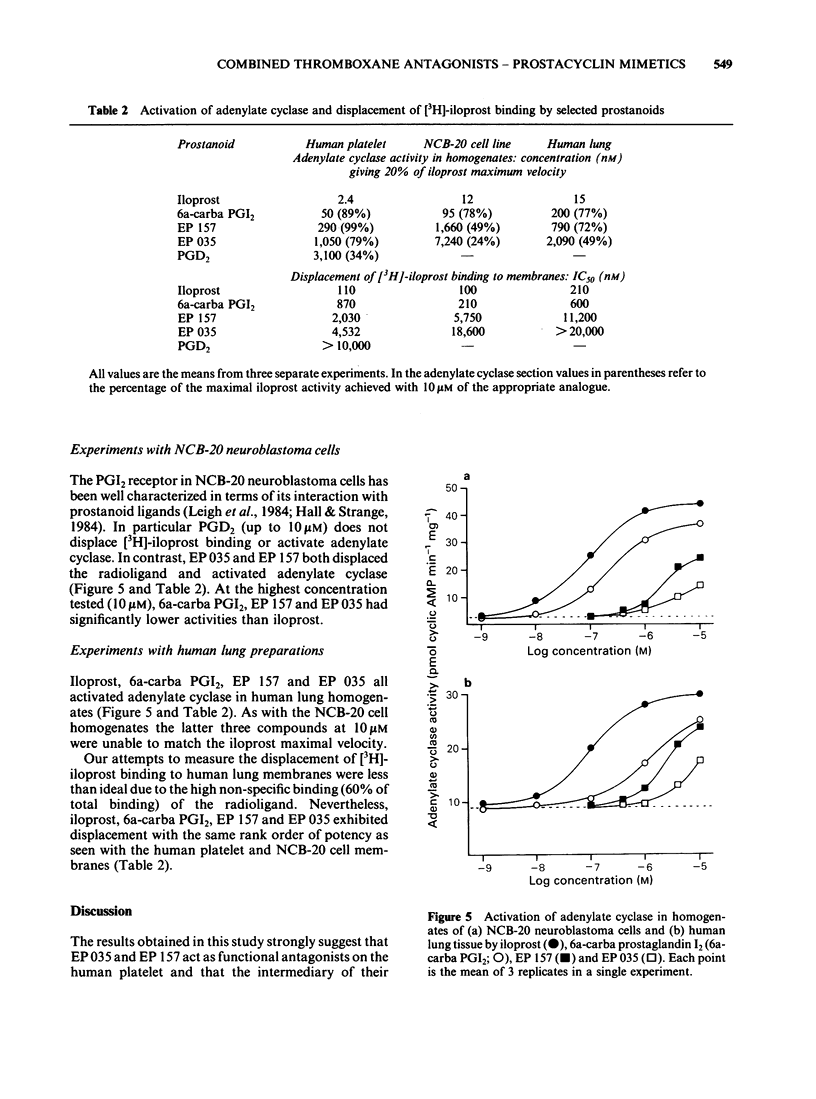
Two prostaglandin endoperoxide analogues, EP 035 and EP 157, behave as specific thromboxane receptor antagonists on isolated smooth muscle preparations such as rabbit aorta, dog saphenous vein and guinea-pig trachea. However, in human platelet-rich plasma (PRP) they produce an unsurmountable block of aggregation induced by a wide range of agents (ADP, platelet-activating factor, thrombin); this inhibitory profile is typical of that seen with either prostaglandin I2 (PGI2) or PGD2. EP 035 and EP 157 induce large increases in cyclic AMP levels (up to 20 times basal) in human PRP. Simultaneous exposure to PGE1 markedly reduces their effect on cyclic AMP; exposure to PGD2 is much less effective in this respect. The adenylate cyclase inhibitor SQ 22,536 opposes the inhibitory action of EP 035, EP 157, iloprost (a stable PGI2 analogue) and PGD2 on platelet aggregation. However, the xanthone derivative AH 6809 blocks the inhibitory action of PGD2 but does not affect EP 035, EP 157 and PGI2 and its structural analogues. EP 035 and EP 157 displace [3H]-iloprost binding to the PGI2 receptor on human platelet membranes. Displacing ability is ranked as follows: iloprost greater than 6a-carba PGI2 greater than EP 157 greater than EP 035 greater than EP 164 (alpha-dinor derivative of EP 157). This order of potency is the same as that found for activation of adenylate cyclase in homogenates of washed human platelets and for inhibition of aggregation in washed human platelets. The activities of EP 035 and EP 157 were studied in two other systems containing PGI2 receptor-adenylate cyclase complexes, the NCB-20 cell line and human lung tissue.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. A., Jones R. L., Peesapati V., Will S. G., Wilson N. H. Competitive antagonism at thromboxane receptors in human platelets. Br J Pharmacol. 1985 Mar;84(3):595–607. doi: 10.1111/j.1476-5381.1985.tb16139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R. A., Jones R. L., Wilson N. H. Ligand binding to thromboxane receptors on human platelets: correlation with biological activity. Br J Pharmacol. 1983 Aug;79(4):953–964. doi: 10.1111/j.1476-5381.1983.tb10541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R. A., Jones R. L., Wilson N. H. Mechanism of the inhibition of platelet aggregation produced by prostaglandin F2 alpha. Prostaglandins. 1985 Apr;29(4):601–610. doi: 10.1016/0090-6980(85)90083-8. [DOI] [PubMed] [Google Scholar]
- Blair I. A., MacDermot J. The binding of [3H]-prostacyclin to membranes of a neuronal somatic hybrid. Br J Pharmacol. 1981 Mar;72(3):435–441. doi: 10.1111/j.1476-5381.1981.tb10994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick F., Gorman R., Bundy G., Honohan T., McGuire J., Sun F. 9,11-Iminoepoxyprosta-5,13-dienoic acid is a selective thromboxane A2 synthetase inhibitor. Biochim Biophys Acta. 1979 May 25;573(2):238–244. doi: 10.1016/0005-2760(79)90057-2. [DOI] [PubMed] [Google Scholar]
- Hall J. M., Strange P. G. The use of a prostacyclin analogue, [3H]iloprost, for studying prostacyclin-binding sites on human platelets and neuronal hybrid cells. Biosci Rep. 1984 Nov;4(11):941–948. doi: 10.1007/BF01116892. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. N., Asaad M. M., Phillips M. B., Goldenberg H. J., Antonaccio M. J. Inhibition of adenylate cyclase in human blood platelets by 9-substituted adenine derivatives. J Cyclic Nucleotide Res. 1979;5(2):125–134. [PubMed] [Google Scholar]
- Jones R. L., Peesapati V., Wilson N. H. Antagonism of the thromboxane-sensitive contractile systems of the rabbit aorta, dog saphenous vein and guinea-pig trachea. Br J Pharmacol. 1982 Jul;76(3):423–438. doi: 10.1111/j.1476-5381.1982.tb09236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leigh P. J., Cramp W. A., MacDermot J. Identification of the prostacyclin receptor by radiation inactivation. J Biol Chem. 1984 Oct 25;259(20):12431–12436. [PubMed] [Google Scholar]
- Miller O. V., Gorman R. R. Evidence for distinct prostaglandin I2 and D2 receptors in human platelets. J Pharmacol Exp Ther. 1979 Jul;210(1):134–140. [PubMed] [Google Scholar]
- Miller O. V., Johnson R. A., Gorman R. R. Inhibition of PGE1-stimulated cAMP accumulation in human platelets by thromboxane a2. Prostaglandins. 1977 Apr;13(4):599–609. doi: 10.1016/0090-6980(77)90231-3. [DOI] [PubMed] [Google Scholar]
- Moncada S., Bunting S., Mullane K., Thorogood P., Vane J. R., Raz A., Needleman P. Imidazole: a selective inhibitor of thromboxane synthetase. Prostaglandins. 1977 Apr;13(4):611–618. doi: 10.1016/0090-6980(77)90232-5. [DOI] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Arachidonic acid metabolites and the interactions between platelets and blood-vessel walls. N Engl J Med. 1979 May 17;300(20):1142–1147. doi: 10.1056/NEJM197905173002006. [DOI] [PubMed] [Google Scholar]


