Abstract
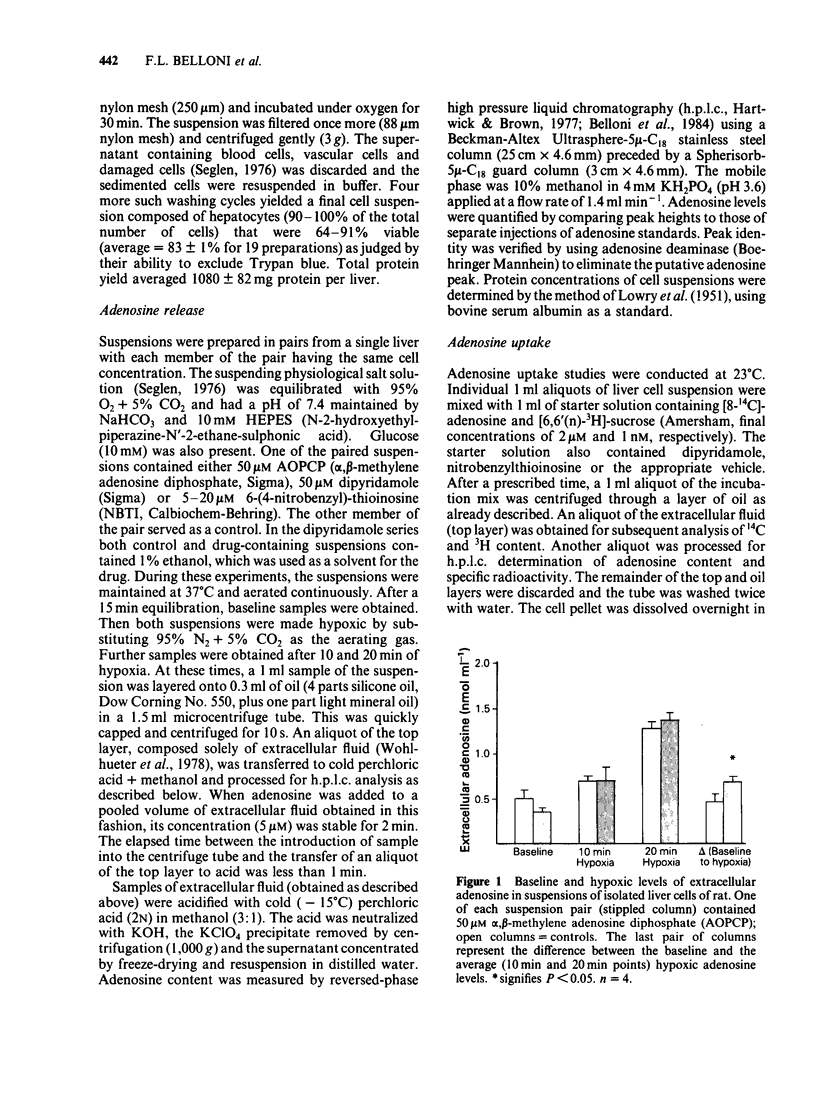
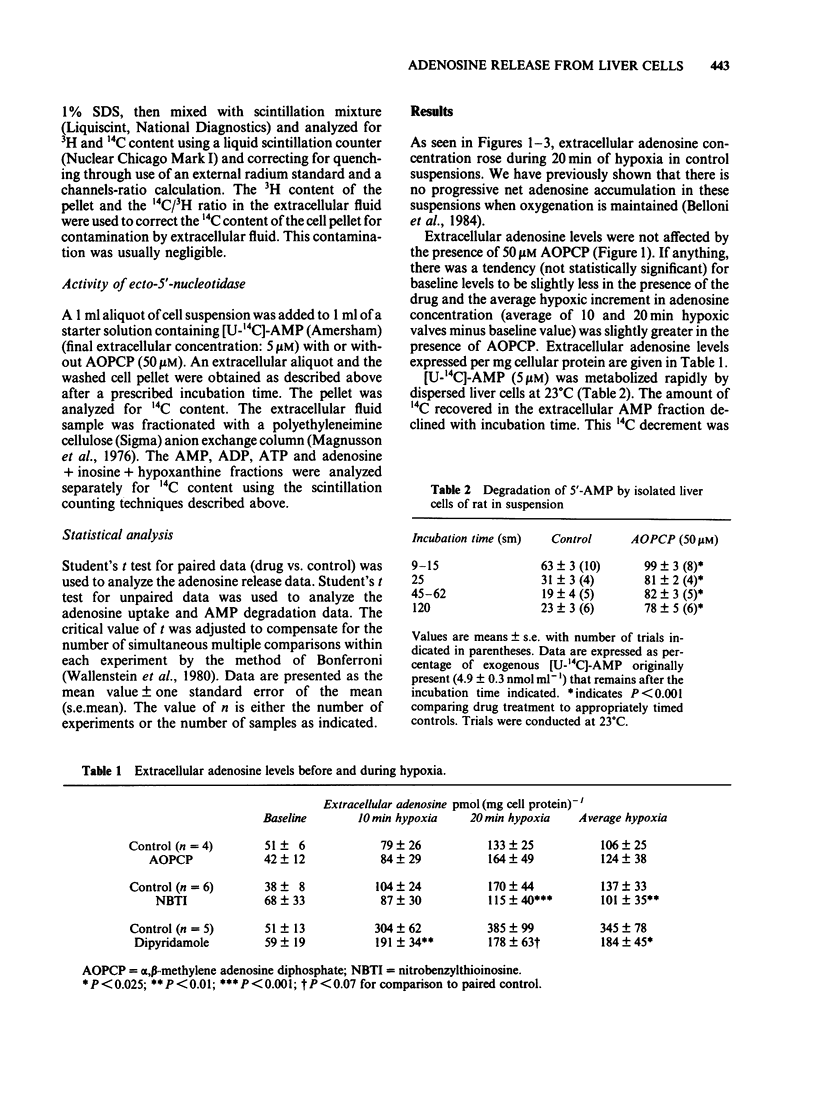
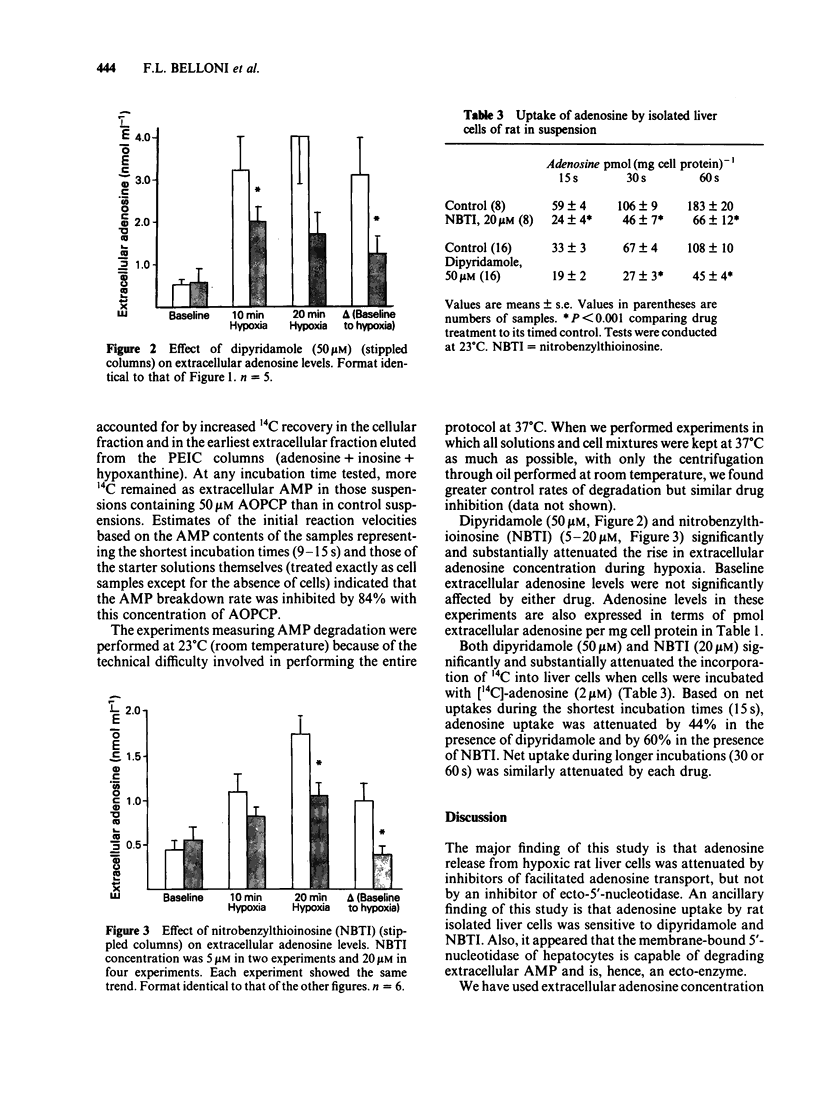
Uptake of [14C]-adenosine into freshly dispersed rat hepatocytes was inhibited 44% by dipyridamole (50 microM) and 60% by nitrobenzylthioinosine (NBTI, 20 microM). The results are consistent with the known ability of these drugs to inhibit adenosine transport in other cell types. The nucleotide analogue, alpha, beta-methylene adenosine diphosphate (AOPCP, 50 microM), inhibited by 84% the degradation of exogenous 5' AMP that occurred rapidly when this substrate alone was presented to isolated hepatocytes. This confirms the ecto-5'-nucleotidase inhibitory properties of this analogue in isolated hepatocytes. During hypoxic incubation, isolated hepatocytes released adenosine, which accumulated in the extracellular volume. Dipyridamole and NBTI each markedly attenuated this extracellular adenosine accumulation. In contrast, AOPCP had no inhibitory effect on net hypoxic adenosine release. It is concluded that hypoxic rat hepatocytes produce adenosine intracellularly and that this adenosine is released via facilitated diffusion to the extracellular space, based on the inhibition observed with the transport inhibitors. The plasma membrane enzyme ecto-5'-nucleotidase does not appear to participate in hypoxic adenosine release from these cells as indicated by the lack of effect of the nucleotidase inhibitor, AOPCP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belloni F. L., Rubio R., Berne R. M. Intracellular adenosine in isolated rat liver cells. Pflugers Arch. 1984 Jan;400(1):106–108. doi: 10.1007/BF00670544. [DOI] [PubMed] [Google Scholar]
- Berne R. M. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980 Dec;47(6):807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Daly J. W., Snyder S. H. Adenosine receptors in brain membranes: binding of N6-cyclohexyl[3H]adenosine and 1,3-diethyl-8-[3H]phenylxanthine. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5547–5551. doi: 10.1073/pnas.77.9.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleit H., Conklyn M., Stebbins R. D., Silber R. Function of 5'-nucleotidase in the uptake of adenosine from AMP by human lymphocytes. J Biol Chem. 1975 Dec 10;250(23):8889–8892. [PubMed] [Google Scholar]
- Frick G. P., Lowenstein J. M. Studies of 5'-nucleotidase in the perfused rat heart. Including measurements of the enzyme in perfused skeletal muscle and liver. J Biol Chem. 1976 Oct 25;251(20):6372–6378. [PubMed] [Google Scholar]
- Frick G. P., Lowenstein J. M. Vectorial production of adenosine by 5'-nucleotidase in the perfused rat heart. J Biol Chem. 1978 Feb 25;253(4):1240–1244. [PubMed] [Google Scholar]
- Hartwick R. A., Brown P. R. Selective analysis for adenosine using reversed-phase high-pressure liquid chromatography. J Chromatogr. 1977 Jul 1;143(4):383–389. doi: 10.1016/s0378-4347(00)80984-6. [DOI] [PubMed] [Google Scholar]
- Knabb R. M., Gidday J. M., Ely S. W., Rubio R., Berne R. M. Effects of dipyridamole on myocardial adenosine and active hyperemia. Am J Physiol. 1984 Nov;247(5 Pt 2):H804–H810. doi: 10.1152/ajpheart.1984.247.5.H804. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Magnusson R. P., Portis A. R., Jr, McCarty R. E. Quantitative, analytical separation of adenine nucleotides by column chromatography on polyethyleneimine-coated cellulose. Anal Biochem. 1976 May 7;72:653–657. doi: 10.1016/0003-2697(76)90580-7. [DOI] [PubMed] [Google Scholar]
- Newby A. C., Holmquist C. A. Adenosine production inside rat polymorphonuclear leucocytes. Biochem J. 1981 Nov 15;200(2):399–403. doi: 10.1042/bj2000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard J. B., O'Connor N., Oliver J. M., Berlin R. D. Uptake and supply of purine compounds by the liver. Am J Physiol. 1975 Oct;229(4):967–972. doi: 10.1152/ajplegacy.1975.229.4.967. [DOI] [PubMed] [Google Scholar]
- Schrader J., Nees S., Gerlach E. Evidence for a cell surface adenosine receptor on coronary myocytes and atrial muscle cells. Studies with an adenosine derivative of high molecular weight. Pflugers Arch. 1977 Jul 19;369(3):251–257. doi: 10.1007/BF00582192. [DOI] [PubMed] [Google Scholar]
- Schütz W., Schrader J., Gerlach E. Different sites of adenosine formation in the heart. Am J Physiol. 1981 Jun;240(6):H963–H970. doi: 10.1152/ajpheart.1981.240.6.H963. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Wohlhueter R. M., Marz R., Graff J. C., Plagemann P. G. A rapid-mixing technique to measure transport in suspended animal cells: applications to nucleoside transport in Novikoff rat hepatoma cells. Methods Cell Biol. 1978;20:211–236. doi: 10.1016/s0091-679x(08)62020-8. [DOI] [PubMed] [Google Scholar]


