Abstract
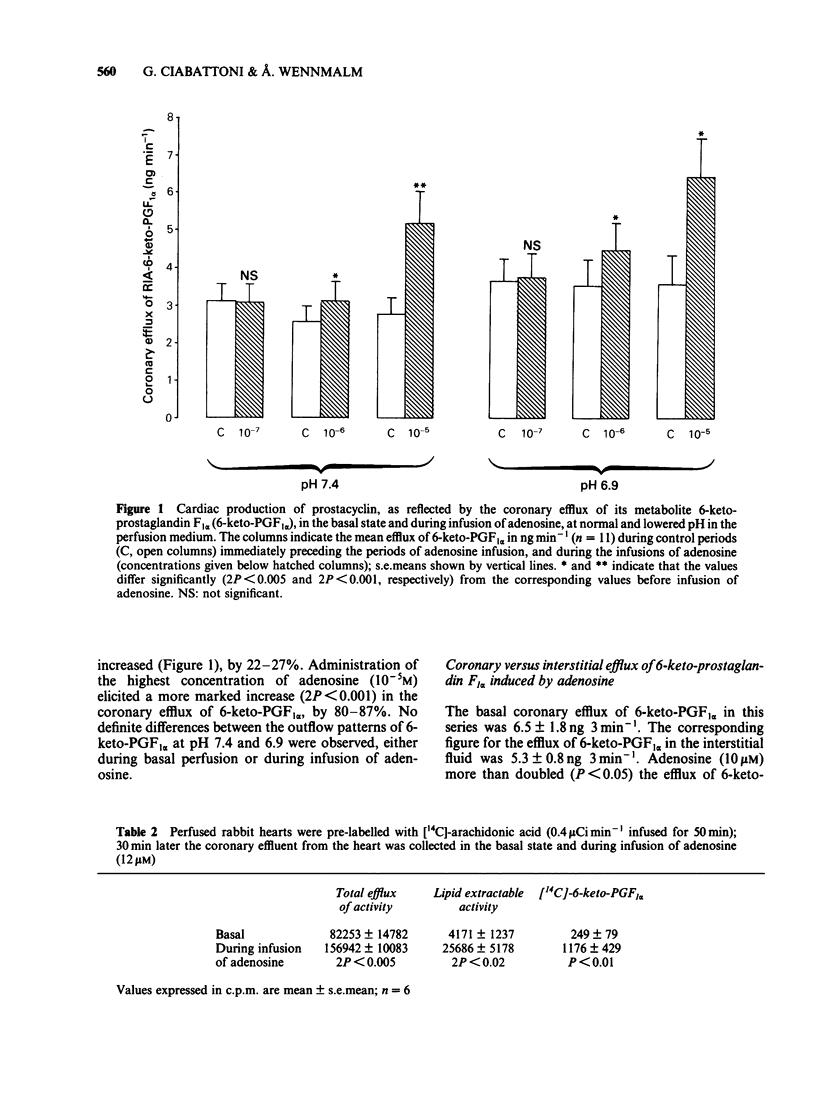
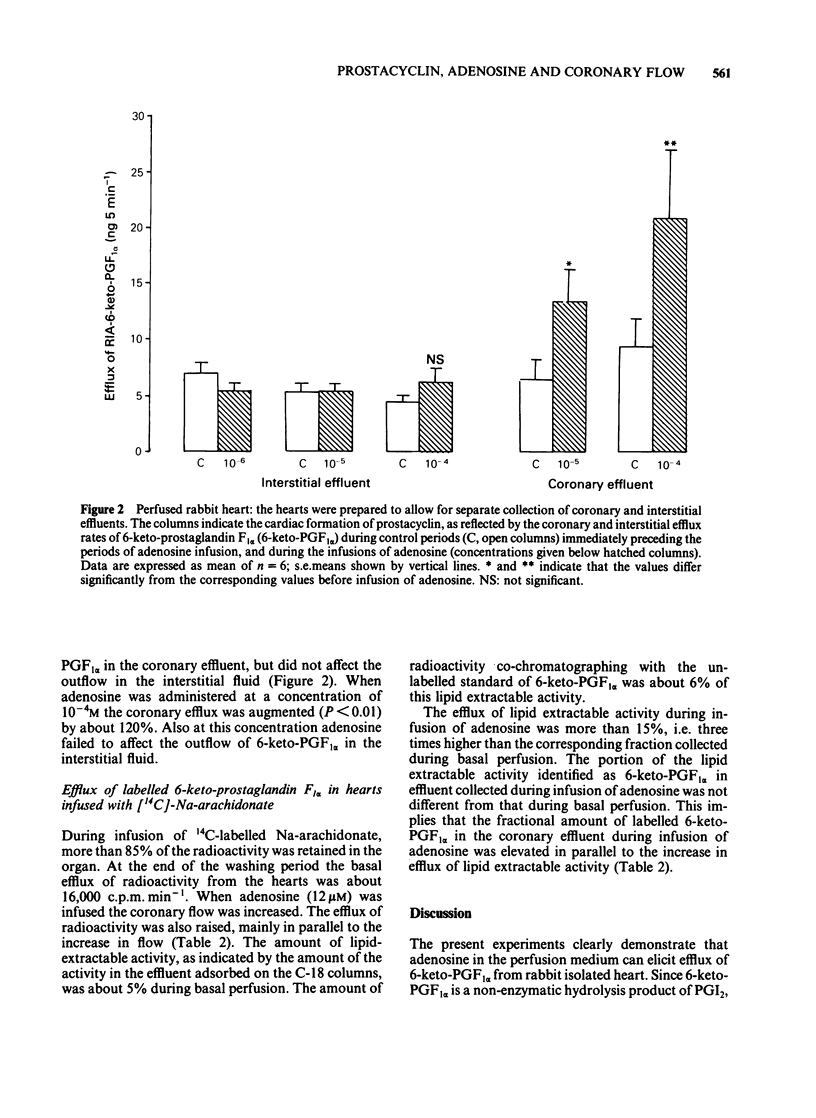
Rabbit hearts were perfused by the Langendorff method with drug-free perfusion medium or with a medium containing adenosine (10(-7) M-10(-4)M) and the coronary and transmyocardial efflux rates of 6-keto-prostaglandin F1 alpha (6-keto-PGF1 alpha) were measured. Perfusion was performed both at pH 7.4 and 6.9. In other experiments the hearts were pre-labelled with [14C]-arachidonic acid and the coronary efflux of radioactivity and of labelled lipids and 6-keto-PGF1 alpha were determined. The basal coronary flow was elevated by almost 70% during tissue acidosis, in comparison to control. Adenosine induced a dose-dependent increase in the coronary flow, amounting to about 75% at normal pH and a drug concentration of 10(-5)M. The adenosine-induced increase in coronary flow was not facilitated by low pH. The base coronary efflux of 6-keto-PGF1 alpha from the hearts was 2.5--3.6 ng min-1. Adenosine (10(-6)-10(-5)M) significantly facilitated this efflux, up to 6.5 ng min-1. The efflux of 6-keto-PGF1 alpha was not changed by perfusion with acidic medium, either in the basal state or during perfusion with adenosine. The basal interstitial efflux of 6-keto-PGF1 alpha was 4.5-5.5 ng 3 min-1. This efflux was not affected by perfusion of the heart with adenosine-containing medium. In hearts pre-labelled with [14C]-arachidonic acid, adenosine (10 microM) induced a specific liberation of labelled lipid-extractable substances, including 6-keto-PGF1 alpha.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell G. J., Flower R. J. Inhibition of phospholipase. Br Med Bull. 1983 Jul;39(3):260–264. doi: 10.1093/oxfordjournals.bmb.a071830. [DOI] [PubMed] [Google Scholar]
- De Deckere E. A., Ten Hoor P. A modified Langendorff technique for metabolic investigations. Pflugers Arch. 1977 Jul 29;370(1):103–105. doi: 10.1007/BF00707954. [DOI] [PubMed] [Google Scholar]
- Edlund A., Fredholm B. B., Patrignani P., Patrono C., Wennmalm A., Wennmalm M. Release of two vasodilators, adenosine and prostacyclin, from isolated rabbit hearts during controlled hypoxia. J Physiol. 1983 Jul;340:487–501. doi: 10.1113/jphysiol.1983.sp014775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder D. R., Belardinelli L., Sperelakis N., Rubio R., Berne R. M. Differential effects of adenosine and nitroglycerin on the action potentials of large and small coronary arteries. Circ Res. 1979 Feb;44(2):176–182. doi: 10.1161/01.res.44.2.176. [DOI] [PubMed] [Google Scholar]
- Herlihy J. T., Bockman E. L., Berne R. M., Rubio R. Adenosine relaxation of isolated vascular smooth muscle. Am J Physiol. 1976 May;230(5):1239–1243. doi: 10.1152/ajplegacy.1976.230.5.1239. [DOI] [PubMed] [Google Scholar]
- IMAI S., RILEY A. L., BERNE R. M. EFFECT OF ISCHEMIA ON ADENINE NUCLEOTIDES IN CARDIAC AND SKELETAL MUSCLE. Circ Res. 1964 Nov;15:443–450. doi: 10.1161/01.res.15.5.443. [DOI] [PubMed] [Google Scholar]
- Isakson P. C., Raz A., Needleman P. Selective incorporation of 14C-arachidonic acid into the phospholipids of intact tissues and subsequent metabolism to 14C-prostaglandins. Prostaglandins. 1976 Nov;12(5):739–748. doi: 10.1016/0090-6980(76)90049-6. [DOI] [PubMed] [Google Scholar]
- Kukovetz W. R., Pöch G., Holzmann S., Wurm A., Rinner I. Role of cyclic nucleotides in adenosine-mediated regulation of coronary flow. Adv Cyclic Nucleotide Res. 1978;9:397–409. [PubMed] [Google Scholar]
- Kunze H., Vogt W. Significance of phospholipase A for prostaglandin formation. Ann N Y Acad Sci. 1971 Apr 30;180:123–125. doi: 10.1111/j.1749-6632.1971.tb53191.x. [DOI] [PubMed] [Google Scholar]
- MacIntyre D. E., Pearson J. D., Gordon J. L. Localisation and stimulation of prostacyclin production in vascular cells. Nature. 1978 Feb 9;271(5645):549–551. doi: 10.1038/271549a0. [DOI] [PubMed] [Google Scholar]
- Marcus A. J., Weksler B. B., Jaffe E. A. Enzymatic conversion of prostaglandin endoperoxide H2 and arachidonic acid to prostacyclin by cultured human endothelial cells. J Biol Chem. 1978 Oct 25;253(20):7138–7141. [PubMed] [Google Scholar]
- Merrill G. F., Haddy F. J., Dabney J. M. Adenosine, theophylline, and perfusate pH in the isolated, perfused guinea pig heart. Circ Res. 1978 Feb;42(2):225–229. doi: 10.1161/01.res.42.2.225. [DOI] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs E. A., Vane J. R. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977 Jan 1;1(8001):18–20. doi: 10.1016/s0140-6736(77)91655-5. [DOI] [PubMed] [Google Scholar]
- Patrono C., Pugliese F., Ciabattoni G., Patrignani P., Maseri A., Chierchia S., Peskar B. A., Cinotti G. A., Simonetti B. M., Pierucci A. Evidence for a direct stimulatory effect of prostacyclin on renin release in man. J Clin Invest. 1982 Jan;69(1):231–239. doi: 10.1172/JCI110435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper P., Vane J. The release of prostaglandins from lung and other tissues. Ann N Y Acad Sci. 1971 Apr 30;180:363–385. doi: 10.1111/j.1749-6632.1971.tb53205.x. [DOI] [PubMed] [Google Scholar]
- Wennmalm A. Nicotine inhibits hypoxia- and arachidonate-induced release of prostacyclin-like activity in rabbit hearts. Br J Pharmacol. 1980 Aug;69(4):545–549. doi: 10.1111/j.1476-5381.1980.tb07901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Deckere E. A., Nugteren D. H., Ten Hoor F. Prostacyclin is the major prostaglandin released from the isolated perfused rabbit and rat heart. Nature. 1977 Jul 14;268(5616):160–163. doi: 10.1038/268160a0. [DOI] [PubMed] [Google Scholar]


