Abstract
The productive infection of human monocyte-derived macrophages (Mφ) by HIV was suppressed by primary CD8+ cells from asymptomatic HIV-infected individuals. This anti-HIV response was noncytotoxic; removal of the CD8+ cells from the infected Mφ leads to virus production. CD8+ cells inhibited HIV replication when separated from the infected Mφ by a transwell filter insert, indicating a diffusible factor made by the CD8+ cells suppressed productive infection of Mφ. Three β-chemokines, which can be secreted by activated CD8+ cells, RANTES (regulated on activation normal T cell expressed and secreted), macrophage inflammatory protein (MIP)-1α and MIP-1β prevented HIV replication in the Mφ cultures. In addition, incubation of acutely infected Mφ with a mixture of neutralizing antibodies to RANTES, MIP-1α, and MIP-1β enhanced virus replication. Nevertheless, neutralization of β-chemokines with specific antibodies did not abolish the suppression by CD8+ cells of HIV replication in Mφ. Thus, even though β-chemokines decrease HIV replication in Mφ, these cytokines are not responsible for the ability of CD8+ cells to inhibit HIV production in these cells.
Monocyte-derived macrophages (Mφ) can be productively infected by HIV (1, 2) and are thought to be one of the first cell types infected during primary infection (3). These cells can continually spread the infection to CD4+ T cells (4). Because HIV infection of Mφ rarely leads to cytopathology, these cells may be a source of increasing viremia when CD4+ cells are decreased in function and number during the later stages of disease (5). Infection with HIV can lead to the reduced capacity of Mφ to present antigen by altering their ability to produce proinflammatory cytokines (6, 7). Moreover, HIV infection of Mφ can alter the effector functions of these cells, such as antibody-dependent cellular cytotoxicity and killing of intracellular organisms (8, 9). Thus, preventing the productive infection of Mφ may be important in limiting the spread of HIV infection and maintaining a functional cellular immune response in the infected individual.
The ability of the cellular immune response to control HIV infection in primary Mφ has received little attention. CD8+ cells are considered a major component of the anti-HIV immunity directed at inhibiting replication of HIV in CD4+ T-cells. Both cytotoxic and noncytotoxic CD8+ cell anti-HIV responses have been observed (10). In the latter case, CD8+ cells control HIV-infected cells not by killing the infected CD4+ cells, but by suppressing the replication of HIV via production of a soluble CD8+ cell antiviral factor (11). Various cytokines produced by CD8+ cells can suppress HIV replication (12). One group of cytokines that has been given much attention are the chemoattractant cytokines (chemokines) (13), which can prevent HIV entry into the cell (14). The C-C or β-chemokine class [RANTES (regulated on activation normal T cell expressed and secreted), macrophage inflammatory protein (MIP)-1α, and MIP-1β] can block macrophage-tropic (M-tropic) viruses from replicating in peripheral blood mononuclear cells (PBMC) and CD4+ cells (15, 16).
We investigated the possibility that CD8+ cells, perhaps through β-chemokine production, control the productive infection of Mφ by M-tropic viruses. The results demonstrate that primary CD8+ cells can inhibit virus replication in Mφ by a noncytotoxic mechanism through the production of a soluble antiviral factor. In addition, the data provide evidence that HIV replication in Mφ can be inhibited by β-chemokines. Despite this finding, the β-chemokines are not involved in the ability of the primary CD8+ cells to suppress the replication of HIV in Mφ.
MATERIALS AND METHODS
Isolation of Macrophages from PBMC.
Mφ were isolated according to the method described elsewhere (17). Briefly, fresh PBMC were obtained by Ficoll-Hypaque (Sigma) gradient centrifugation of peripheral blood from HIV-seronegative donors (18). The PBMC (50 × 106) were added to 75-cm2 flasks (Falcon) coated with 2% gelatin (Sigma) followed by autologous plasma and incubated for 45 min at 37°C. Nonadherent cells were removed, and the adherent cells were washed five times with RPMI 1640 medium (BioWhittaker). The adherent cells were removed from the flasks by incubating the cells in 5 mM EDTA (Sigma). The cells were then washed three times with calcium and magnesium-free PBS (BioWhittaker). Mφ isolated by this technique were found to be >98% nonspecific esterase positive by using the α-naphtyl acetate assay (Sigma).
Acute Infection of Macrophages.
Mφ (2 × 106) were cultured for 7 days in 6-well plates (Falcon) containing 2 ml of serum-free macrophage medium (SFMM; Life Technologies, Inc., Gaithersburg, MD) to allow for differentiation. In some studies, Mφ were exposed to 100 units/ml of recombinant human granulocyte macrophage colony stimulatory factor (GM-CSF; Genzyme) during the 7-day incubation period. The cells then were treated with 2 μg/ml of polybrene (Sigma) for 30 min in SFMM. Polybrene-treated cells were washed once in calcium and magnesium-free PBS and infected with 1 ml of 2,500 × TCID50 of HIV-1SF162 or HIV-1SF128A/106 cells for 2 hr. These strains are M-tropic and nonsyncytium-inducing viruses isolated in our laboratory and grown only in PBMC (19). The volume of SFMM was increased to 2 ml and allowed to incubate overnight. The next day the infected Mφ were washed with calcium and magnesium-free PBS and treated with 0.05% trypsin and 0.2% versene in saline (Life Technologies) for 2 min. RPMI 1640 medium with 10% heat-inactivated (56°C, 30 min) human AB+ serum (BioWhittaker) was added to the infected macrophages for 10 min to quench the trypsin. This procedure was followed by two washes with calcium and magnesium-free PBS. These steps were performed to ensure that the level of HIV particles in the culture fluid measured 3 days later was not caused by the presence of residual virus bound nonspecifically to the cell surface during acute infection (20). The infected Mφ were cultured in 2 ml of SFMM.
Measurement of Antiviral Activity of CD8+ Cells.
The CD8+ cells were obtained from the peripheral blood of HIV-infected individuals who are asymptomatic, have CD4+ cell counts above 500 cells/μl, and are not taking antiretroviral medication. For recovery of CD8+ cells, the PBMC from HIV-infected donors, isolated by standard procedures (18), were stimulated with phytohemagglutinin (3 μg/ml; Sigma) and anti-CD28 antibody (1 μg/ml, Immunotech, Westbrook, ME) for 3 days in RPMI 1640 medium with 10% heat-inactivated fetal bovine serum (Gemini Biological Products, Calabasas, CA) supplemented with 2 mM glutamine (BioWhittaker), 100 units/ml penicillin (BioWhittaker), 100 μg/ml streptomycin (BioWhittaker), and 100 units/ml recombinant human interleukin 2 (Collaborative Biomedical Products, Bedford, MA). CD8+ cells from the activated PBMC subsequently were obtained by using immunomagnetic beads as described previously (21).
In measuring the anti-HIV activity of CD8+ cells, the infected macrophages were cultured in RPMI 1640 medium with 10% human AB+ serum, 2 mM glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 100 units/ml recombinant human interleukin 2 in the absence or presence of 2 × 106 CD8+ cells. In some studies the CD8+ cells were separated from the infected Mφ by a transwell membrane (0.45 μm) 6-well insert (Corning-Costar). Mφ were always below the insert, whereas the CD8+ cells were placed in the insert. These inserts did not affect the viability of the Mφ or the ability of HIV to replicate in these cells. Fluids from the transwell membrane device, representing a mixture of the supernatants from the upper and lower compartments, were removed every 3 days and monitored for particle-associated reverse transcriptase (RT) activity (22) and β-chemokine levels (see below).
Recombinant β-Chemokine and Neutralizing Antibodies.
Recombinant human β-chemokines RANTES, MIP-1α, and MIP-1β, and their neutralizing polyclonal goat antibodies were obtained from R&D Systems. Control antibodies were normal goat IgG (R&D Systems). The concentration and source of antibodies used to neutralize the β-chemokines in this study also were able to block the ability of 100 ng/ml of recombinant human RANTES, MIP-1α, and MIP-1β to inhibit the replication of non-syncytium-inducing virus strains in CD4+ cells (16). RANTES, MIP-1α, and MIP-1β levels in the culture fluids were measured by β-chemokine-specific ELISA kits and used according to the methods outlined by the manufacturer (R&D Systems).
RESULTS
Suppression of HIV Replication in Macrophages by CD8+ Cells.
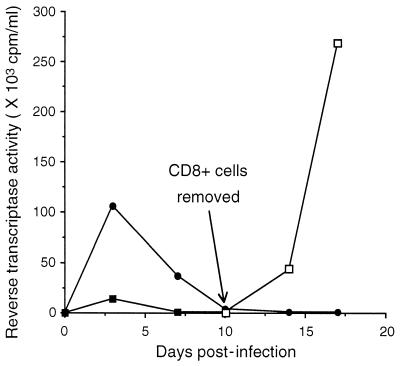
The ability of purified CD8+ cells from HIV-infected individuals to suppress HIV replication in acutely infected blood-derived Mφ was determined. Fig. 1 is an example of two separate studies in which cocultures of CD8+ cells at a 1:1 cell ratio with acutely infected Mφ resulted in the reduction of RT activity by 87% (110 × 103 cpm/ml of RT activity in the absence of CD8+ cells versus 14 × 103 cpm/ml in the presence of CD8+ cells). Six days after peak RT activity, the nonadherent CD8+ cells were removed, and the presence of virus in the culture fluid of the remaining adherent cells was measured. After the removal of CD8+ cells, the RT activity in the culture fluid increased from 1 × 103 cpm/ml to 42 × 103 cpm/ml, and later rose to 270 × 103 cpm/ml (Fig. 1).
Figure 1.
Effect of CD8+ cells from an HIV-infected individual on HIV replication in macrophages. Mφ were isolated from PBMC of an HIV-seronegative donor, allowed to differentiate for 7 days, and then infected with HIV-1SF162. Infected Mφ were cultured in the absence (•) or presence (▪) of an equal number of activated CD8+ cells from an HIV-infected donor. Cell-free culture fluids were collected every 3 days and monitored for particle-associated RT activity. CD8+ cells were removed from the coculture 6 days after peak RT activity, and the remaining adherent cells were monitored for virus production (□). The data are representative of two separate experiments giving similar results.
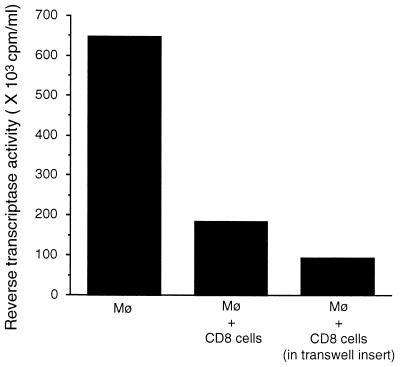
To determine whether the ability of CD8+ cells to suppress HIV replication was mediated by a soluble factor, the infected Mφ were separated from the CD8+ cells by a transwell filter insert that allowed for the macromolecular exchange of culture fluids between the cells in the separate chambers. In the cell contact control culture, the RT activity of the culture fluids of the infected macrophages mixed with the CD8+ cells decreased from 650 × 103 cpm/ml to 190 × 103 cpm/ml (71% suppression). Importantly, the CD8+ cells in the transwell insert over the infected macrophages also were able to inhibit virus replication [RT activity = 92 × 103 cpm/ml (86% suppression)] (Fig. 2).
Figure 2.
Ability of CD8+ cells to suppress HIV replication when separated from the infected macrophages by a transwell insert. Mφ were isolated from the PBMC of an HIV-seronegative donor, allowed to differentiate for 7 days and then infected with HIV-1SF162. The infected Mφ were cultured in the absence or presence of activated CD8+ cells from an HIV-infected donor. The CD8+ cells were either cocultured directly with the infected Mφ or added to a transwell (0.45 μm filter) insert, which was placed over the infected Mφ. Cell-free culture fluids were collected every 3 days and monitored for particle-associated RT activity. The results were obtained during peak RT activity in the control group. The data are representative of two separate experiments giving similar results.
Productive Infection of Macrophage in the Presence of β-Chemokines.
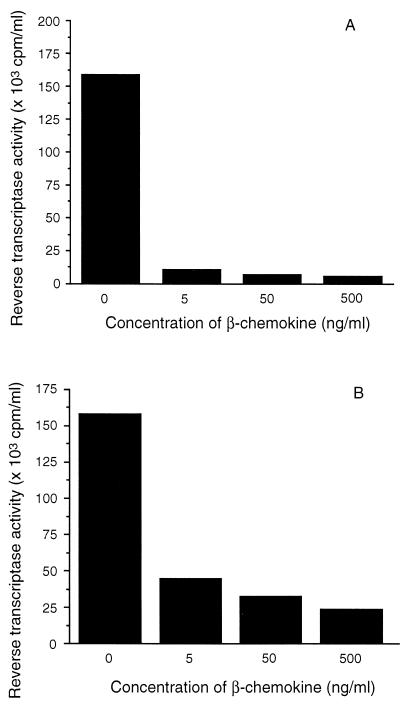
Because β-chemokines are capable of suppressing M-tropic HIV strains and are produced by CD8+ cells that can suppress HIV replication, we next examined the effect of recombinant human β-chemokines on acute HIV infection of Mφ. Mφ were exposed to a mixture of RANTES, MIP-1α, and MIP-1β during infection with HIV-1SF162, and the virus levels were monitored. Infected Mφ from one subject, when exposed to 5, 50, and 500 ng/ml of RANTES, MIP-1α, and MIP-1β showed a decrease in the level of RT activity. The RT activity of the fluids from the control Mφ culture was 160 × 103 cpm/ml, which was reduced in the treated cultures to ≤ 11 × 103 cpm/ml (93% suppression) (Fig. 3A) A similar dose-dependent effect of the β-chemokines was observed with Mφ from another subject (Fig. 3B). This influence of β-chemokines on virus production in Mφ was not limited to the HIV-1SF162 strain. The RT activity of culture fluids from Mφ infected with HIV-1SF128A and treated with 500 ng/ml of β-chemokines was decreased from 160 × 103 cpm/ml observed in the control culture to 18 × 103 cpm/ml (89% suppression). Thus, the β-chemokines RANTES, MIP-1α, and MIP-1β used together can reduce the productive infection of Mφ by HIV.
Figure 3.
Effect of the β-chemokines, RANTES, MIP-1α, and MIP-1β on HIV infection of macrophages. Mφ were isolated from the PBMC of two HIV-seronegative donors (A and B), allowed to differentiate for 7 days, and then infected with HIV-1SF162. The cultures were maintained in serum-free Mφ medium in the absence or presence of a mixture of 5, 50, or 500 ng/ml of each of the β-chemokines, RANTES, MIP-1α, and MIP-1β. Cell-free culture fluids were collected every 3 days and monitored for particle-associated RT activity. The data represent results of three experiments and were obtained during peak RT activity in the control group.
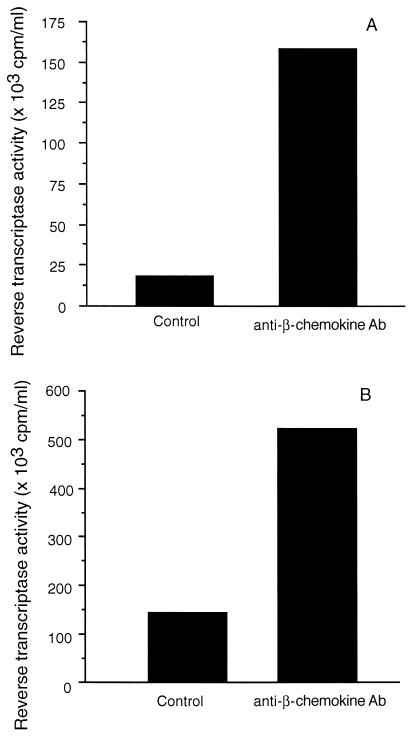
To further examine the sensitivity of HIV replication in Mφ to the effects of β-chemokines, acutely infected cells were treated with anti-RANTES, anti-MIP-1α, and anti-MIP-1β neutralizing antibodies. Treatment with the anti-β-chemokine antibodies increased RT activity in the culture fluids of infected Mφ from one subject by 9-fold (18 × 103 to 170 × 103 cpm/ml; Fig. 4A) and from another subject by 4-fold (150 × 103 to 530 × 103 cpm/ml; Fig. 4B). The levels of RANTES, MIP-1α, and MIP-1β in the culture fluids of the control Mφ cultures were often as high as 1,500 pg/ml whereas the culture fluids of infected Mφ incubated with neutralizing antibodies to the β-chemokines all were below the level of detection of the ELISA.
Figure 4.
Effect of neutralizing antibodies to RANTES, MIP-1α, and MIP-1β on the ability of HIV to infect macrophages. Macrophages were isolated from PBMC from two HIV-seronegative donors (A and B) and allowed to differentiate for 7 days. They then were infected with HIV-1SF162 and maintained in serum-free (Mφ) medium in the absence or presence of an antibody mixture containing 100 μg/ml of anti-RANTES, 50 μg/ml of anti-MIP-1α, and 100 μg/ml of anti-MIP-1β polyclonal goat neutralizing antibodies (Ab). Cell-free culture fluids were collected every 3 days and monitored for particle-associated RT activity. The data are representative of two experiments and were obtained during peak RT activity in the control group.
Role of β-Chemokines in the Ability of CD8+ Cells to Suppress Productive HIV Infection of Macrophages.
The possible role of β-chemokines in the suppression of HIV replication in Mφ by CD8+ cells was examined. A mixture of neutralizing antibodies to the β-chemokines was added to the transwell insert containing CD8+ cells that were above the infected Mφ in the culture plate (Table 1). The level of RT activity of Mφ from two different individuals was reduced from 530 and 730 × 103 cpm/ml to 130 and 190 × 103 cpm/ml (75 and 74% suppression, respectively) by CD8+ cells from two different asymptomatic HIV-infected subjects, respectively. This level of suppression was not reduced by the presence of anti-β-chemokine antibodies [100 and 150 × 103 cpm/ml of RT activity, respectively (81% and 79% suppression, respectively)] despite the substantial decrease in the level of β-chemokines in the culture fluids (Table 1).
Table 1.
Effect of anti-RANTES, MIP-1α, and MIP-1β neutralizing antibodies on the ability of CD8+ cells to suppress HIV replication when separated from the infected Mφ by a transwell insert
| Exp. | GM-CSF | CD8+ cells* | Treatment | RANTES,† pg/ml | MIP-1α,† pg/ml | MIP-1β,† pg/ml | RT activity, ×10−3 cpm/ml |
|---|---|---|---|---|---|---|---|
| 1 | − | − | No antibody | <5 | 681 | 796 | 533.6 |
| − | +‡ | No antibody | <5 | 87.5 | 115.2 | 130.5 | |
| − | + | Control antibody§ | 247.1 | 269 | 220 | 158.1 | |
| − | + | anti-β-chemokine antibody¶ | <5 | <6 | 14.6 | 102.4 | |
| 2 | − | − | No antibody | <5 | 964.2 | 1453 | 729.1 |
| − | + | No antibody | 25.7 | 201 | 26.6 | 186.5 | |
| − | + | Control antibody | 26.5 | 571.7 | 714.8 | 72.7 | |
| − | + | anti-β-chemokine antibody | <5 | <6 | <4 | 152.8 | |
| 3 | + | − | No antibody | <5 | 180.4 | 27.1 | 1305.0 |
| + | + | No antibody | 126.9 | 190.4 | 114.1 | 267.1 | |
| + | + | anti-β-chemokine antibody | <5 | 6.4 | 6.1 | 99.0 |
Separated from macrophages by a transwell insert (0.45 μm pore size filter).
β-chemokine levels were determined by RANTES, MIP-1α, and MIP-1β specific ELISA.
The level of RANTES, MIP-1α, and MIP-1β in the culture fluid of CD8+ cells in the absence of infected macrophages was 249.5, 24.9, and 20.3 pg/ml, respectively.
250 μg/ml of normal goat IgG.
100 μg/ml of anti-RANTES, 50 μg/ml of anti-MIP-1α, and 100 μg/ml of anti-MIP-1β polyclonal goat antibodies.
To determine whether CD8+ cells could suppress high-level HIV replication in Mφ we exposed the cells to GM-CSF before acute infection and the addition of the CD8+ cells. The results indicated that CD8+ cells from an asymptomatic individual were able to suppress HIV replication in macrophages treated with GM-CSF [from an RT activity of 1,300 × 103 cpm/ml to 270 × 103 cpm/ml (79% suppression)] (Table 1). The addition of antibodies to β-chemokines reduced the levels of RANTES from 126.9 to <5 pg/ml, of MIP-1α from 190.4 to 6 pg/ml, and of MIP-1β from 114.1 to 6 pg/ml, but did not decrease the ability of the CD8+ cells to suppress HIV replication in these cells [RT activity was 99 × 103 cpm/ml (92% suppression)].
DISCUSSION
These studies were undertaken to examine whether HIV infection of Mφ can be controlled by cellular immune responses. The results demonstrate that primary CD8+ cells from HIV-infected individuals suppress the productive infection of Mφ. This inhibition of virus replication is noncytotoxic (Fig. 1) and appears to be mediated by a soluble factor (Fig. 2). The studies also demonstrate that β-chemokines can block HIV replication in Mφ (Figs. 3 and 4) but the antiviral effect of the soluble factor secreted by the CD8+ cells (Fig. 2) is independent of these cytokines (Table 1).
CD8+ cells from HIV-infected individuals suppress HIV replication in CD4+ T lymphocytes by using a noncytotoxic mechanism associated with the production of a soluble CD8+ cell antiviral factor (11, 12). A similar process, therefore, appears present when CD8+ cells are cultured with HIV-infected Mφ. The continual presence of CD8+ cells is required because removal of these cells led to virus production (Fig. 1), as described previously with infected CD4+ cells (23). These findings support the conclusion that HIV-infected cells are not eliminated from the culture. Moreover, separation of the Mφ from the CD8+ cells by a transwell insert did not reduce the ability of the CD8+ cells to suppress productive infection of Mφ (Fig. 2 and Table 1). This observation indicates that contact is not required for the control of HIV replication in Mφ by CD8+ cells; a diffusible factor made by the CD8+ cells appears responsible for suppressing the productive infection of Mφ.
The ability of CD8+ cells to inhibit HIV replication in Mφ has not been studied extensively. One report (24) demonstrated that culture fluids from Herpesvirus saimiri-transformed CD8+ cells from HIV-infected individuals were capable of suppressing infection of Mφ. Although these transformed cells presumably allow for a constant source of CD8+ cell antiviral factor (25), the finding does not necessarily reflect the events occurring with primary CD8+ cells in which a variety of cytokines could be involved.
With respect to the nature of the anti-HIV factors produced by CD8+ cells, RANTES, MIP-1α, and MIP-1β together have been shown to inhibit the replication of M-tropic but not T-tropic viruses in PBMC and CD4+ lymphocytes (15). Our studies indicate that low concentrations of recombinant β-chemokines are also effective in suppressing the replication of M-tropic viruses in Mφ (Fig. 3). The β-chemokines used together were the most effective in controlling virus replication in Mφ although RANTES, MIP-1α, and MIP-1β added alone also could reduce virus replication by at least 50% (data not shown). The potential role of natural β-chemokines in controlling HIV replication in Mφ also was demonstrated in our studies. When β-chemokines were removed from the culture fluids of acutely infected Mφ with neutralizing antibodies, virus replication increased (Fig. 4). This in vitro finding suggests that naturally produced β-chemokines can regulate HIV production in Mφ.
Controversy exists over whether infection of Mφ by M-tropic viruses is sensitive to β-chemokines. Despite the expression of this chemokine receptor on Mφ, some initial studies suggested that the β-chemokines do not block this infection (24, 26) and might even enhance it (27). In contrast, recent studies have demonstrated that HIV infection of Mφ can be blocked by RANTES, MIP-1α, and MIP-1β (28). The present studies addressed this issue and confirmed the ability of β-chemokines to block infection of Mφ by M-tropic HIV-1 strains. Importantly, our studies and those of others (28) demonstrate that the level of virus produced can be controlled by not only the addition of recombinant β-chemokines in combination but also the naturally produced β-chemokines from the infected Mφ (Fig. 4).
The reason for the discrepancies among the different studies is unclear. The use of an alternative chemokine receptor by M-tropic viruses is not likely because Mφ from individuals who are homozygous for the absence of CCR5 expression are resistant to infection by M-tropic viruses (29). The differences observed could reflect the techniques used to isolate and differentiate the Mφ, variables in media and chemokines used, the time of virus inoculation, or the strain of virus used for infection. However, when Mφ were isolated by plastic adherence and grown in the presence of GM-CSF according to the methods previously described by others (24), the inhibition of productive infection by the M-tropic strains, HIV-1BAL and HIV-1SF162, still could be achieved with β-chemokines (data not shown).
Importantly, in the present study, we demonstrate that CD8+ cells suppress the replication of HIV in Mφ, independent of the β-chemokines (Table 1). Specifically, neutralizing antibodies to RANTES, MIP-1α, and MIP-1β decreased the levels of these chemokines in the culture fluids of infected Mφ to near or at the lowest level of detection for the chemokine-specific ELISA. However, this neutralization of β-chemokines had no effect on the ability of CD8+ cells to suppress HIV replication, even when the amount of virus produced was enhanced (e.g., GM-CSF-treated Mφ) (Table 1). This observation was made when the CD8+ cells were in contact with infected Mφ (data not shown) as well as separated by a transwell membrane insert (Table 1). The results of these studies place further emphasis on the CD8+ cell noncytotoxic response as a major mechanism for controlling HIV replication in infected individuals.
ABBREVIATIONS
- Mφ
monocyte-derived macrophages
- RANTES
regulated on activation normal T cell expressed and secreted
- MIP
macrophage inflammatory protein
- M-tropic
macrophage-tropic
- PBMC
peripheral blood mononuclear cells
- GM-CSF
granulocyte macrophage colony stimulatory factor
- RT
reverse transcriptase
References
- 1.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson J K, Cross G D, Callaway C S, McDougal J S. J Immunol. 1986;137:323–329. [PubMed] [Google Scholar]
- 3.Nuovo G J, Forde A, MacConnell P, Fahrenwald R. Am J Pathol. 1993;143:40–48. [PMC free article] [PubMed] [Google Scholar]
- 4.McElrath M J, Pruett J E, Cohn Z A. Proc Natl Acad Sci USA. 1989;86:675–679. doi: 10.1073/pnas.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orenstein J M, Fox C, Wahl S M. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 6.Lacroix F, Zhao D Y, Izaguirre C A, Filion L G. Clin Immunol Immunopathol. 1993;67:109–116. doi: 10.1006/clin.1993.1052. [DOI] [PubMed] [Google Scholar]
- 7.Denis M, Ghadirian E. AIDS Res Hum Retro. 1994;10:1619–1627. doi: 10.1089/aid.1994.10.1619. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin G C, Fleischmann J, Chung Y, Koyanagi Y, Chen I S, Golde D W. Proc Natl Acad Sci USA. 1990;87:3933–3937. doi: 10.1073/pnas.87.10.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowe S M, Vardaxis N J, Kent S J, Maerz A L, Hewish M J, McGrath M S, Mills J. J Leukocyte Biol. 1994;56:318–327. doi: 10.1002/jlb.56.3.318. [DOI] [PubMed] [Google Scholar]
- 10.Levy J A, Mackewicz C E, Barker E. Immunol Today. 1996;17:217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 11.Walker C M, Levy J A. Immunology. 1989;66:628–630. [PMC free article] [PubMed] [Google Scholar]
- 12.Mackewicz C E, Ortega H, Levy J A. Cell Immunol. 1994;153:329–343. doi: 10.1006/cimm.1994.1032. [DOI] [PubMed] [Google Scholar]
- 13.Strieter R M, Standiford T J, Huffnagle G B, Colletti L M, Lukacs N W, Kunkel S L. J Immunol. 1996;156:3583–3586. [PubMed] [Google Scholar]
- 14.Berger E A. AIDS. 1997;11:s3–s16. [PubMed] [Google Scholar]
- 15.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 16.Mackewicz C E, Barker E, Greco G, Reyes-Teran G, Levy J A. J Clin Invest. 1997;100:921–930. doi: 10.1172/JCI119608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freundlich B, Avdalovic N. J Immunol Methods. 1983;62:31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- 18.Levy J A, Shimabukuro J. J Infect Dis. 1985;152:734–738. doi: 10.1093/infdis/152.4.734. [DOI] [PubMed] [Google Scholar]
- 19.Cheng-Mayer C, Weiss C, Seto D, Levy J A. Proc Natl Acad Sci USA. 1989;80:8575–8579. doi: 10.1073/pnas.86.21.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang S, Levy J A. J Virol Methods. 1991;33:39–46. doi: 10.1016/0166-0934(91)90005-k. [DOI] [PubMed] [Google Scholar]
- 21.Mackewicz C E, Ortega H W, Levy J A. J Clin Invest. 1991;87:1462–1466. doi: 10.1172/JCI115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman A D, Banapour B, Levy J A. Virology. 1985;147:326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- 23.Walker C M, Moody D J, Stites D P, Levy J A. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 24.Moriuchi H, Moriuchi M, Combadiere C, Murphy P M, Fauci A S. Proc Natl Acad Sci USA. 1996;93:15341–15345. doi: 10.1073/pnas.93.26.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackewicz C E, Orque R, Jung J, Levy J A. Clin Immunol Immunopathol. 1997;82:274–281. doi: 10.1006/clin.1996.4292. [DOI] [PubMed] [Google Scholar]
- 26.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, et al. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 27.Schmidtmayerova H, Sherry B, Bukrinsky M. Nature (London) 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- 28.Verani A, Scarlatti G, Comar M, Tresoldi E, Polo S, Giacca M, Lusso P, Siccardi A G, Vercelli D. J Exp Med. 1997;185:805–816. doi: 10.1084/jem.185.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rana S, Besson G, Cook D G, Rucker J, Smyth R J, et al. J Virol. 1997;71:3219–3227. doi: 10.1128/jvi.71.4.3219-3227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]