Abstract
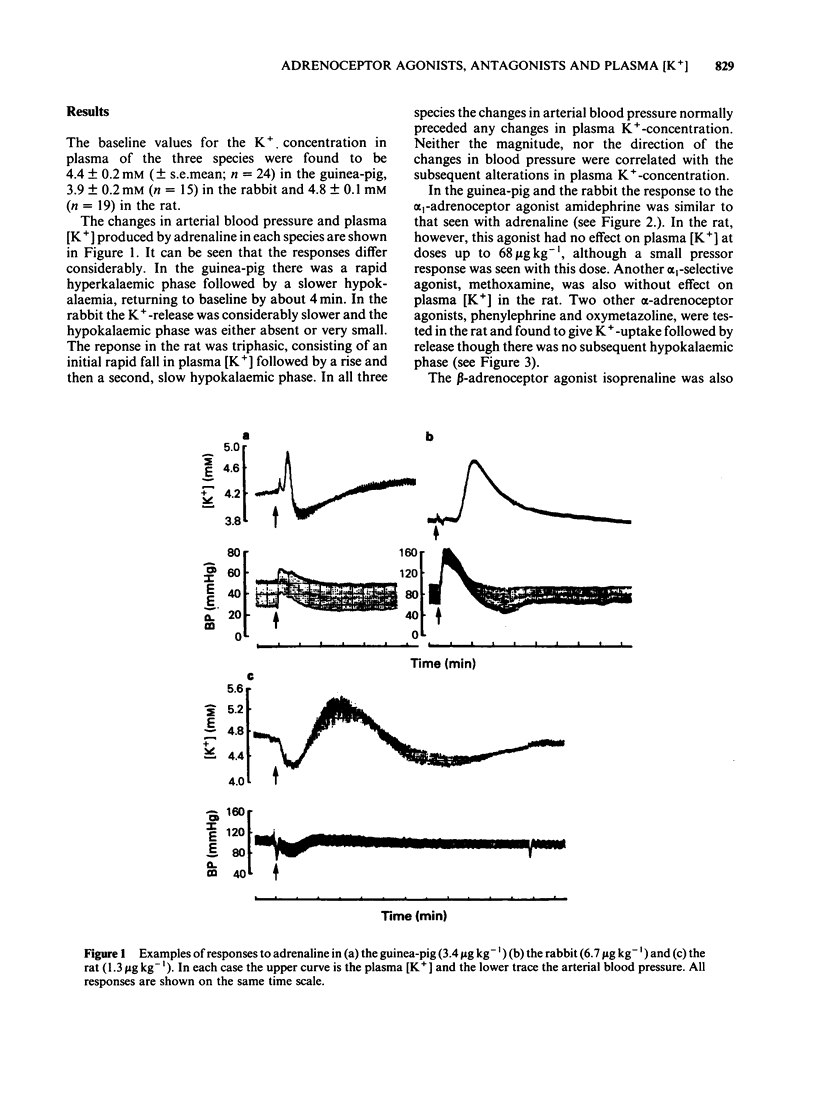
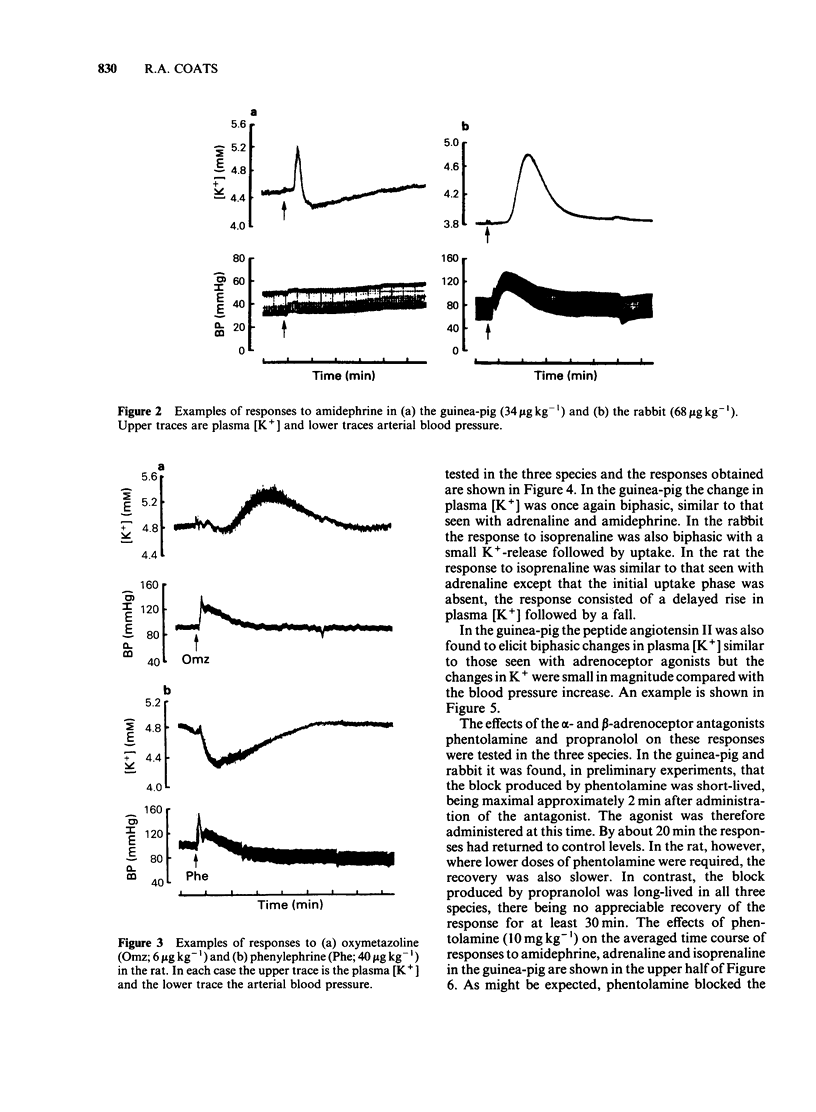
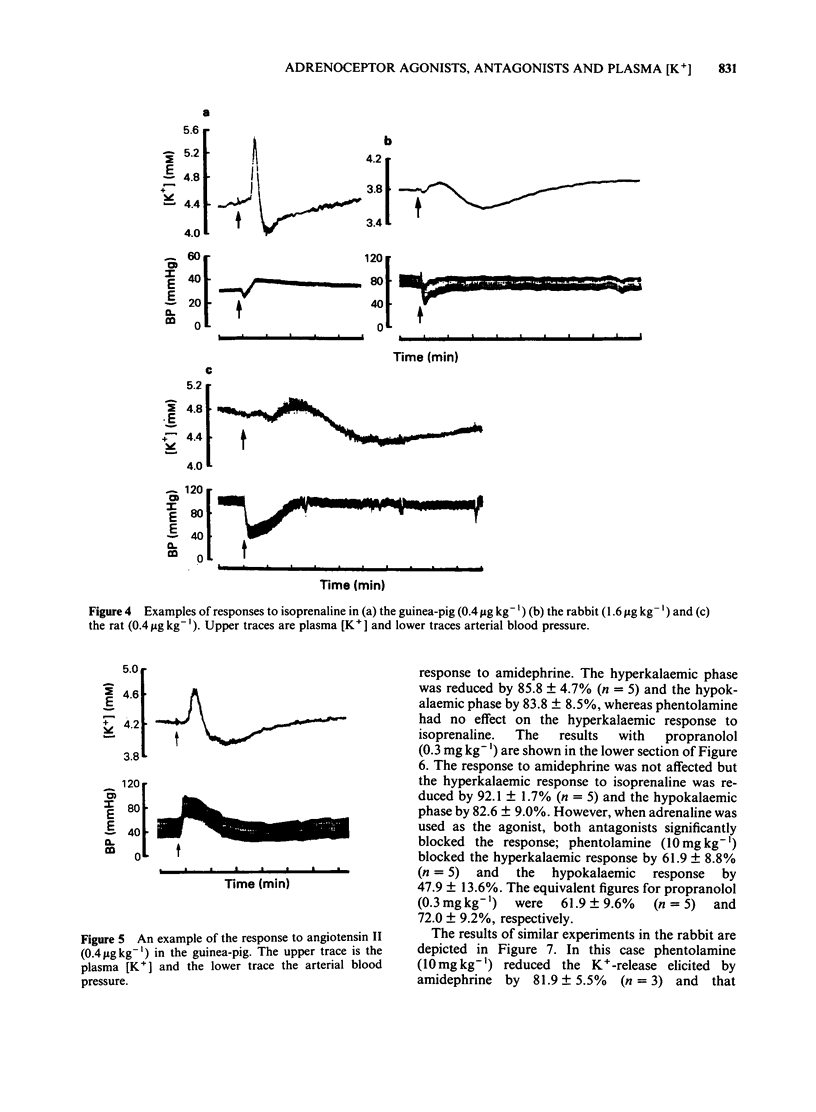
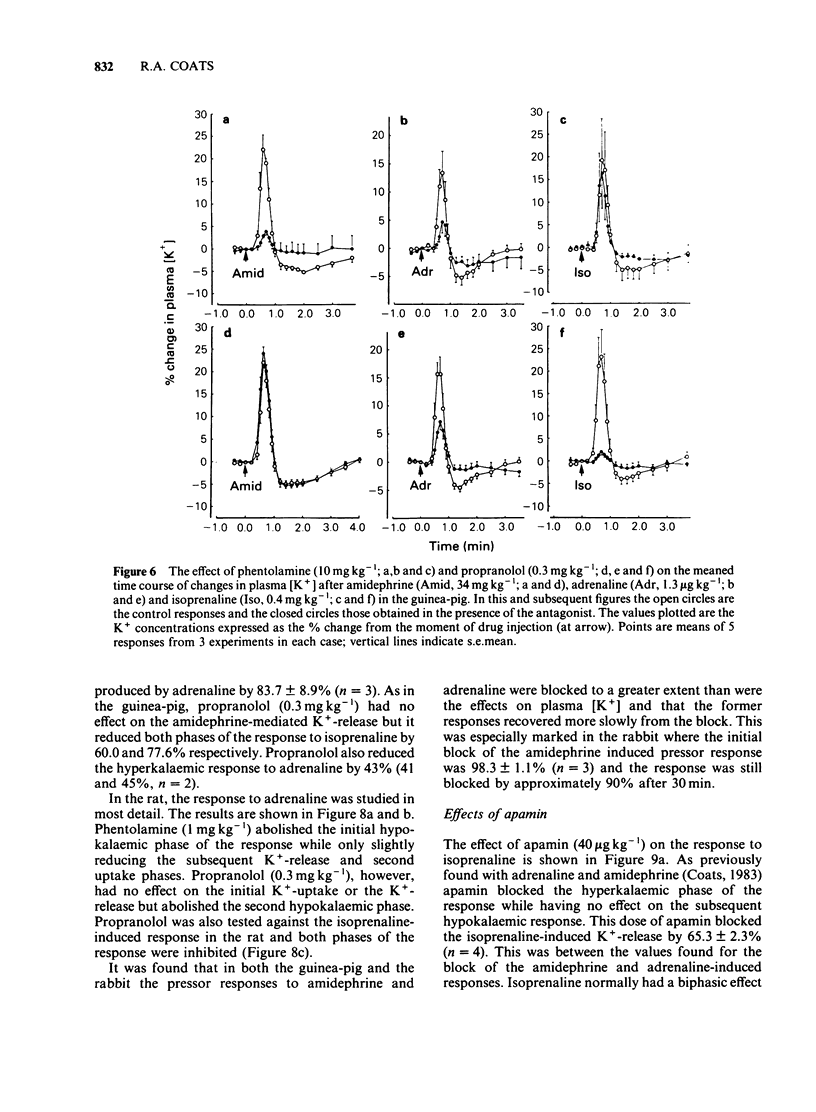
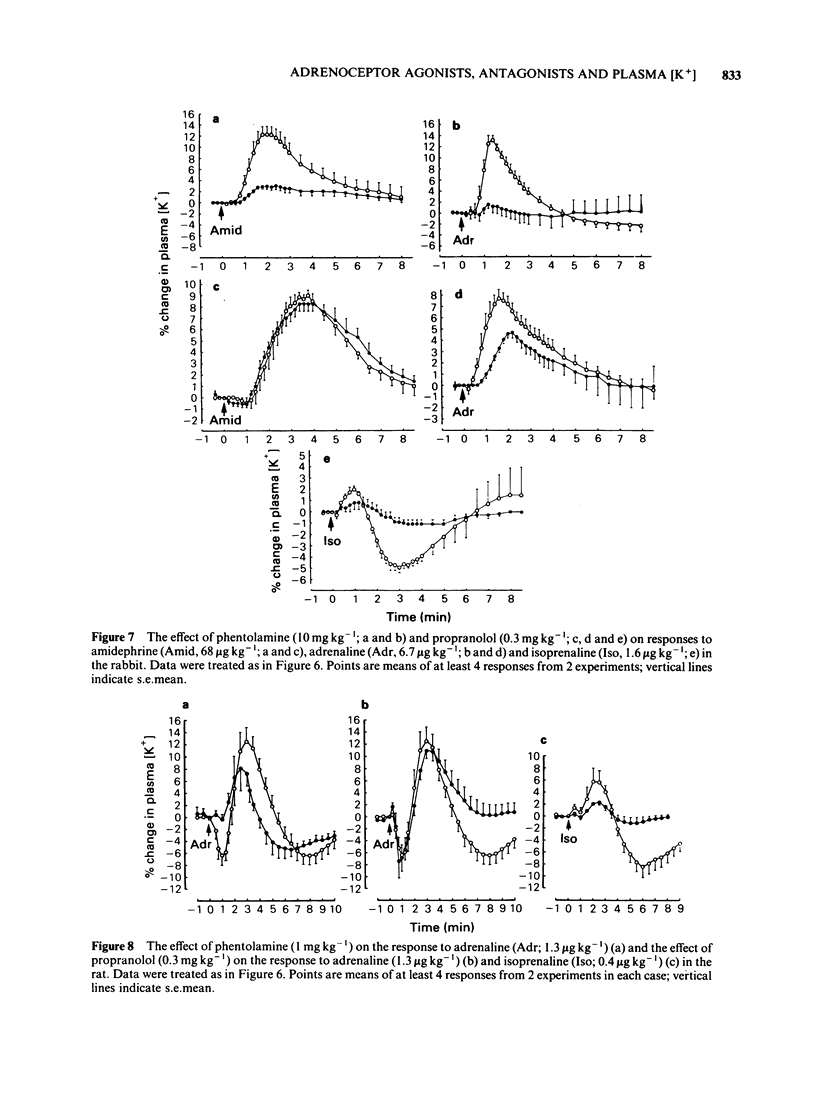
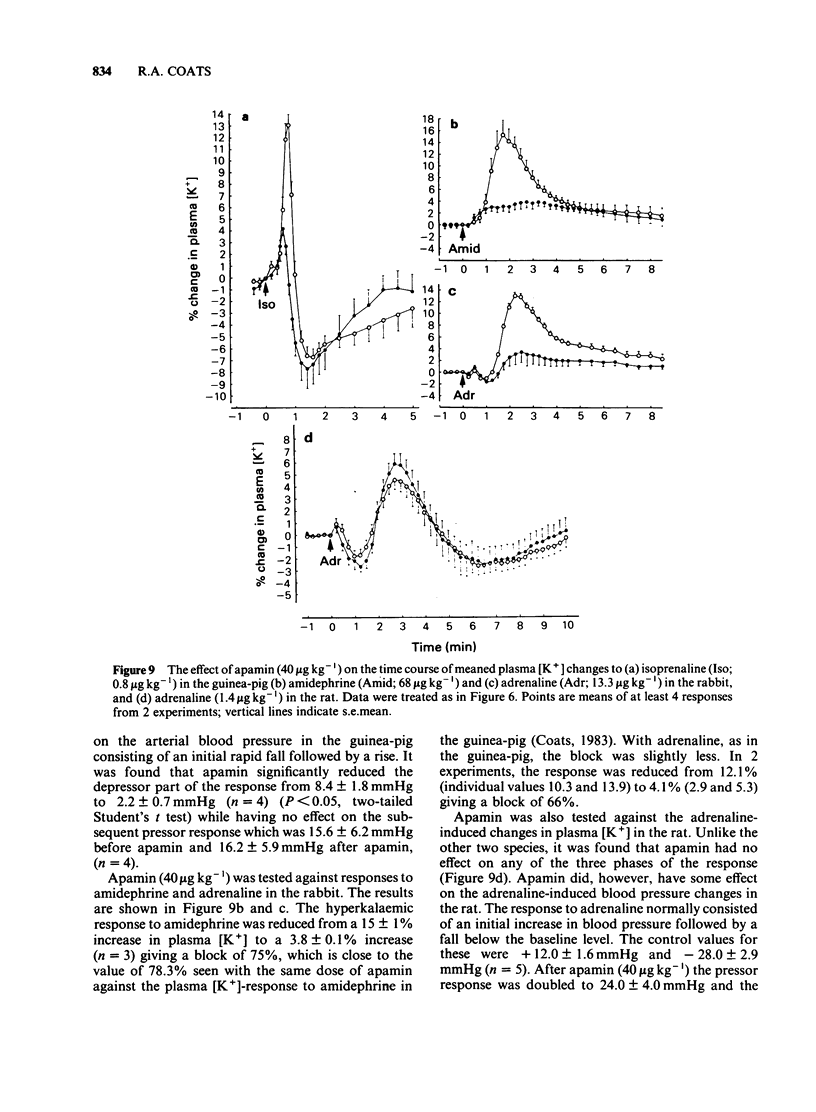
An intravenous K+-sensitive electrode has been used to monitor plasma [K+] changes induced by alpha- and beta-adrenoceptor agonists in anaesthetized guinea-pigs, rabbits and rats. The effects of phentolamine and propranolol on these responses were studied. In the guinea-pig both alpha- and beta-adrenoceptor agonists produced a biphasic response consisting of an initial rapid increase in [K+] which was followed, within 1 min, by a fall below baseline. The antagonist studies indicated that in this species both phases of the response could be elicited by either alpha- or beta-adrenoceptor activation. In the rabbit the responses were both slower and smaller than those seen in the guinea-pig and required larger agonist doses. In addition it was found that the increase in plasma [K+] was alpha-adrenoceptor-mediated while the subsequent fall was seen only with beta-adrenoceptor activation. In the rat triphasic changes in plasma [K+] were seen consisting of an initial decrease which was alpha-adrenoceptor-mediated, followed by an increase and then a second fall which was elicited by beta-adrenoceptor stimulation. The increase in plasma [K+] was only slightly reduced by either alpha- or beta-adrenoceptor antagonists. Apamin, a toxin from bee venom which blocks Ca2+-activated K+-channels, was found to block the hyperkalaemic phase of the response in the guinea-pig and rabbit but had no effect in the rat. It is concluded that there are marked species differences in the effects of adrenoceptor agonists on plasma [K+] in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Becker J., Jakob A. alpha-Adrenergic stimulation of glycolysis and Na+, K+-transport in perfused rat liver. Eur J Biochem. 1982 Nov 15;128(2-3):293–296. doi: 10.1111/j.1432-1033.1982.tb06964.x. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981 Aug;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Giraud F., Poggioli J., Claret M. Alpha-adrenergically mediated changes in membrane lipid fluidity and Ca2/ binding in isolated rat liver plasma membranes. Biochim Biophys Acta. 1983 Jun 23;731(3):387–396. doi: 10.1016/0005-2736(83)90033-0. [DOI] [PubMed] [Google Scholar]
- CRAIG A. B., Jr Observations on epinephrine and glucagon-induced glycogenolysis and potassium loss in the isolated perfused frog liver. Am J Physiol. 1958 May;193(2):425–430. doi: 10.1152/ajplegacy.1958.193.2.425. [DOI] [PubMed] [Google Scholar]
- Capiod T., Berthon B., Poggioli J., Burgess G. M., Claret M. The effect of Ca2+ -mobilising hormones on the Na+ --K+ pump in isolated rat liver hepatocytes. FEBS Lett. 1982 May 3;141(1):49–52. doi: 10.1016/0014-5793(82)80013-6. [DOI] [PubMed] [Google Scholar]
- Castro-Tavares J. A comparison between the influence of pindolol and propranolol on the response of plasma potassium to catecholamines. Arzneimittelforschung. 1976 Feb;26(2):238–241. [PubMed] [Google Scholar]
- Castro-Tavares J. Effects of isoprenaline and phenylephrine on plasma potassium: role of the liver. Arch Int Pharmacodyn Ther. 1975 Nov;218(1):110–119. [PubMed] [Google Scholar]
- Castro-Travares J., Cardoso W. Effects of adrenergic stimulant and blocking drugs on the release and uptake of potassium by the liver. Arch Int Pharmacodyn Ther. 1974 May;209(1):100–112. [PubMed] [Google Scholar]
- Coats R. A. Effects of apamin on alpha-adrenoceptor-mediated changes in plasma potassium in guinea-pigs. Br J Pharmacol. 1983 Nov;80(3):573–580. doi: 10.1111/j.1476-5381.1983.tb10731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks T. M., Jenkinson D. H., Koller K. Interactions between receptors that increase cytosolic calcium and cyclic AMP in guinea-pig liver cells. Br J Pharmacol. 1984 Sep;83(1):281–291. doi: 10.1111/j.1476-5381.1984.tb10144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Silva J. L. The action of adrenaline on serum potassium. J Physiol. 1936 Feb 8;86(2):219–228. doi: 10.1113/jphysiol.1936.sp003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS S., BECKETT S. B. MECHANISM OF THE POTASSIUM MOBILIZING ACTION OF EPINEPHRINE AND GLUCAGON. J Pharmacol Exp Ther. 1963 Dec;142:318–326. [PubMed] [Google Scholar]
- El-Refai M. F., Blackmore P. F., Exton J. H. Evidence for two alpha-adrenergic binding sites in liver plasma membranes. Studies with [3H]epinephrine and [3H]dihydroergocryptine. J Biol Chem. 1979 Jun 10;254(11):4375–4386. [PubMed] [Google Scholar]
- Haylett D. G., Jenkinson D. H. Effects of noradrenaline on potassium reflux, membrane potential and electrolyte levels in tissue slices prepared from guinea-pig liver. J Physiol. 1972 Sep;225(3):721–750. doi: 10.1113/jphysiol.1972.sp009966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob A., Diem S. Metabolic responses of perfused rat livers to alpha- and beta-adrenergic agonists, glucagon and cyclic AMP. Biochim Biophys Acta. 1975 Sep 8;404(1):57–66. doi: 10.1016/0304-4165(75)90147-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson D. H., Koller K. Interactions between the effects of alpha- and beta-adrenoceptor agonists and adenine nucleotides on the membrane potential of cells in guinea-pig liver slices. Br J Pharmacol. 1977 Jan;59(1):163–175. doi: 10.1111/j.1476-5381.1977.tb06991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvam D. C., Riggilo D. A., Lish P. M. Effect of some new beta-adrenergic blocking agents on certain metabolic responses to catecholamines. J Pharmacol Exp Ther. 1965 Aug;149(2):183–192. [PubMed] [Google Scholar]
- Lim M., Linton R. A., Band D. M. Continuous intravascular monitoring of epinephrine-induced changes in plasma potassium. Anesthesiology. 1982 Oct;57(4):272–278. doi: 10.1097/00000542-198210000-00004. [DOI] [PubMed] [Google Scholar]
- O'BRIEN G. S., EID C. H., MURPHY Q. R., Jr, MEEK W. J. Effect of elimination of hepatic circulation on cyclopropane-epinephrine ventricular tachycardia and arterial plasma potassium in dogs. J Pharmacol Exp Ther. 1954 Nov;112(3):374–377. [PubMed] [Google Scholar]
- Todd E. P., Vick R. L. Kalemotropic effect of epinephrine: analysis with adrenergic agonists and antagonists. Am J Physiol. 1971 Jun;220(6):1964–1969. doi: 10.1152/ajplegacy.1971.220.6.1964. [DOI] [PubMed] [Google Scholar]
- Tsujimoto A., Tanino S., Kaniike K., Seto K., Kurogochi Y. Relationship of hyperkalemic response to hepatic phosphorylase activation induced by adrenaline. Jpn J Pharmacol. 1965 Dec;15(4):423–428. doi: 10.1254/jjp.15.423. [DOI] [PubMed] [Google Scholar]
- Vick R. L., Todd E. P., Luedke D. W. Epinephrine-induced hypokalemia: relation to liver and skeletal muscle. J Pharmacol Exp Ther. 1972 Apr;181(1):139–146. [PubMed] [Google Scholar]



