Abstract
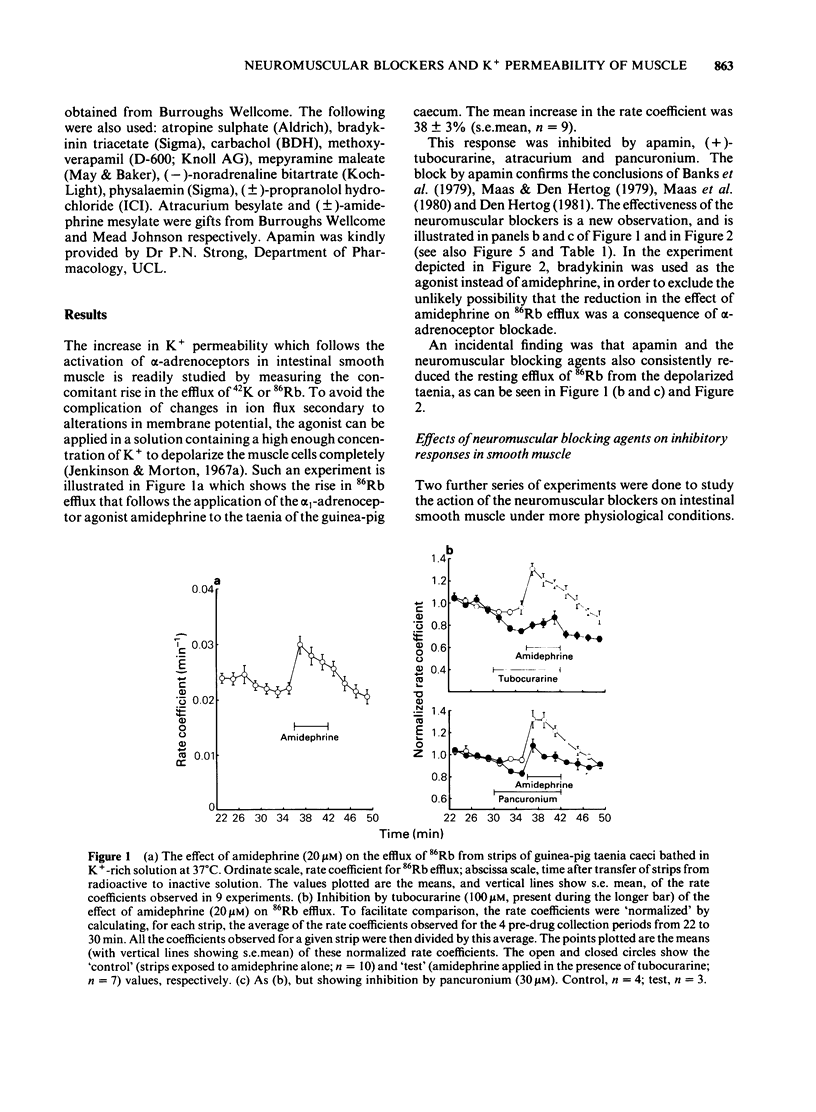
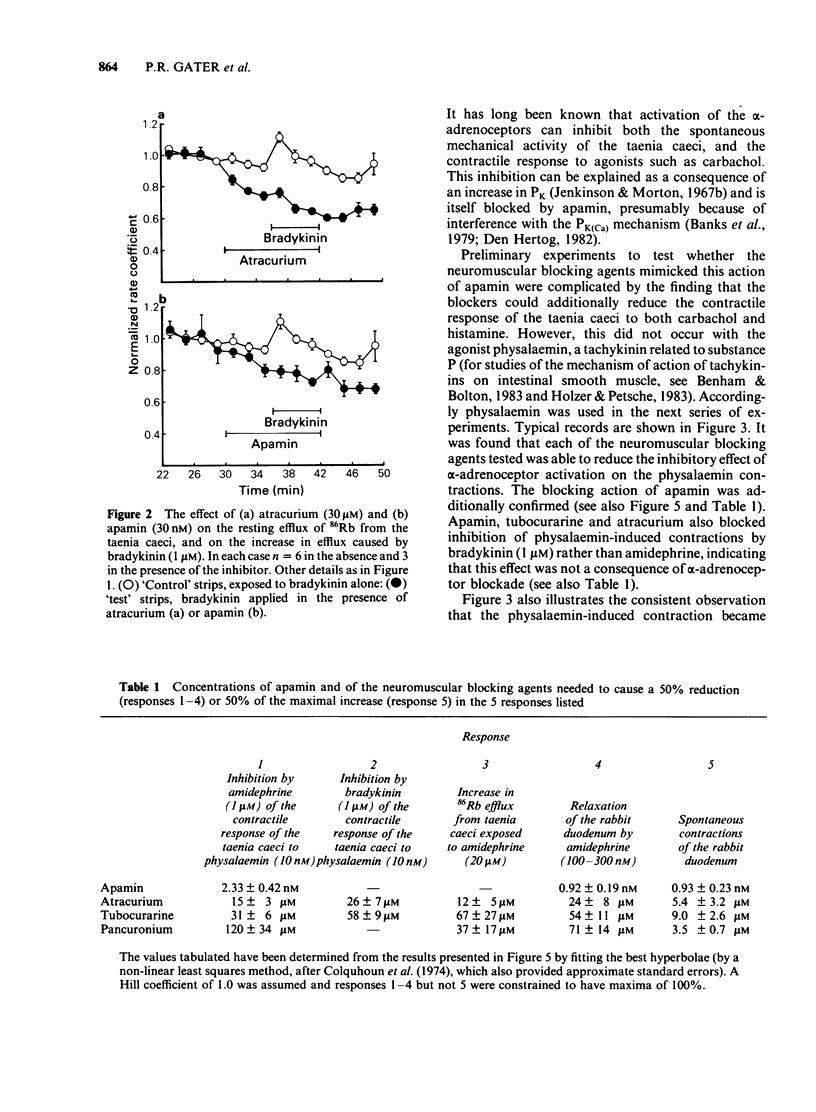
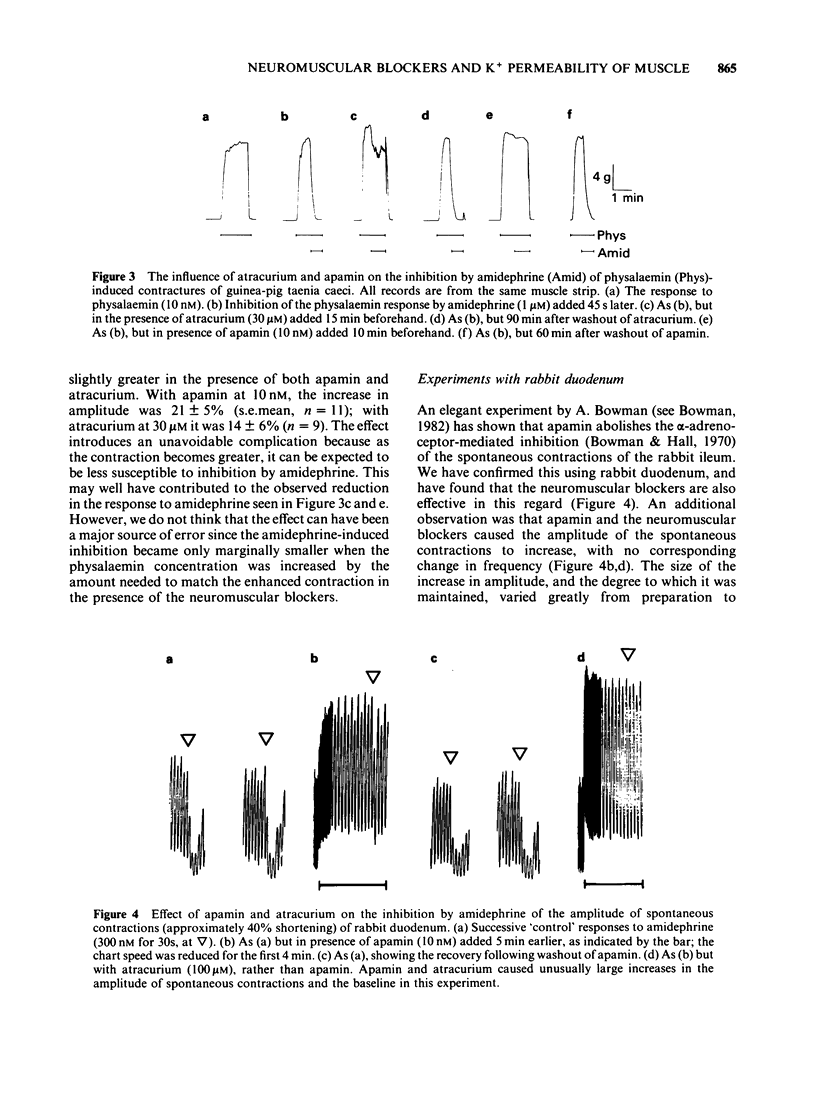
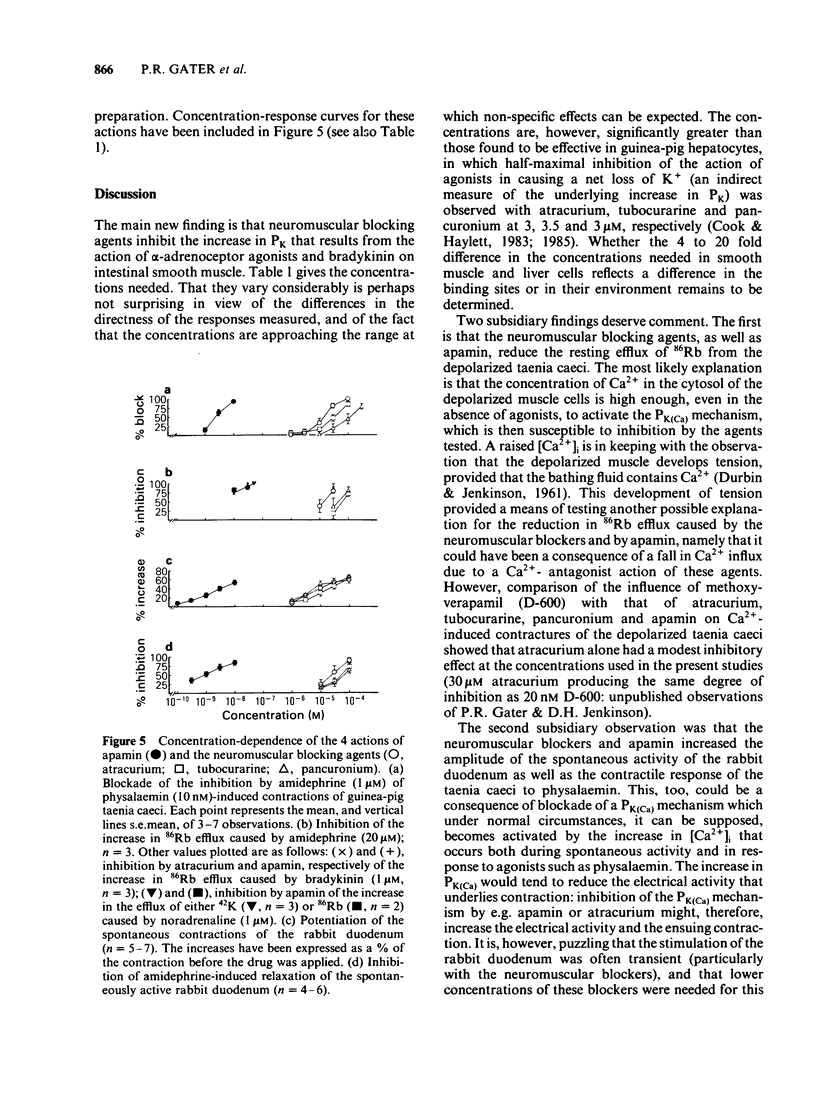
The neuromuscular blocking agents tubocurarine, atracurium and pancuronium have been tested for their ability to inhibit receptor-mediated increases in the K+ permeability of intestinal smooth muscle. All three agents, as well as the bee venom peptide apamin, reduced both the resting efflux of 86Rb and the increase in efflux caused by the application of either bradykinin (1 microM) or an alpha 1-adrenoceptor agonist, amidephrine (20 microM), to depolarized strips of guinea-pig taenia caeci. This suggested that like apamin, the neuromuscular blocking agents inhibit the Ca2+-dependent K+ permeability (PK(Ca] mechanism which in this tissue is activated by a variety of membrane receptors. The concentrations (IC50S) of atracurium, pancuronium and (+)-tubocurarine which reduced the effect of amidephrine on 86Rb efflux by 50% were 12, 37 and 67 microM respectively. Also in keeping with an ability to block PK(Ca), the neuromuscular blockers and apamin reduced the inhibition by amidephrine and bradykinin of physalaemin-mediated contractions of the taenia caeci. The IC50 values were 15, 31 and 120 microM for atracurium, tubocurarine and pancuronium respectively, and 2.3 nM for apamin. Each of the neuromuscular blockers, and apamin, increased the spontaneous contractions of the rabbit duodenum and blocked the inhibitory effect of amidephrine thereon. It is concluded that the PK(Ca) mechanism in the longitudinal smooth muscle of the intestine It is concluded that the PK(Ca) mechanism in the longitudinal smooth muscle of the intestine resembles that of hepatocytes and sympathetic ganglion cells in its susceptibility to inhibition by neuromuscular blocking agents, as well as by apamin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Comparison of the excitatory actions of substance P, carbachol, histamine and prostaglandin F2 alpha on the smooth muscle of the taenia of the guinea-pig caecum. Br J Pharmacol. 1983 Nov;80(3):409–420. doi: 10.1111/j.1476-5381.1983.tb10710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman W. C., Hall M. T. Inhibition of rabbit intestine mediated by alpha- and beta-adrenoceptors. Br J Pharmacol. 1970 Feb;38(2):399–415. doi: 10.1111/j.1476-5381.1970.tb08528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981 Aug;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Suppression of spontaneous spike generation by catecholamines in the smooth muscle of the guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):103–119. doi: 10.1098/rspb.1969.0014. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Rang H. P., Ritchie J. M. The binding of tetrodotoxin and alpha-bungarotoxin to normal and denervated mammalian muscle. J Physiol. 1974 Jul;240(1):199–226. doi: 10.1113/jphysiol.1974.sp010607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. S., Haylett D. G. Effects of apamin, quinine and neuromuscular blockers on calcium-activated potassium channels in guinea-pig hepatocytes. J Physiol. 1985 Jan;358:373–394. doi: 10.1113/jphysiol.1985.sp015556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURBIN R. P., JENKINSON D. H. The calcium dependence of tension development in depolarized smooth muscle. J Physiol. 1961 Jun;157:90–96. doi: 10.1113/jphysiol.1961.sp006707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hertog A. Calcium and the action of adrenaline, adenosine triphosphate and carbachol on guinea-pig taenia caeci. J Physiol. 1982 Apr;325:423–439. doi: 10.1113/jphysiol.1982.sp014160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hertog A. Calcium and the alpha-action of catecholamines on guinea-pig taenia caeci. J Physiol. 1981 Jul;316:109–125. doi: 10.1113/jphysiol.1981.sp013776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedan J. S., Hogaboom G. K., O'Donnell J. P. Comparison of the effects of apamin, a Ca2+-dependent K+ channel blocker, and arylazido aminopropionyl ATP (ANAPP3), a P2-purinergic receptor antagonist, in the guinea-pig vas deferens. Eur J Pharmacol. 1984 Sep 17;104(3-4):327–334. doi: 10.1016/0014-2999(84)90409-6. [DOI] [PubMed] [Google Scholar]
- Feldberg W., Lotti V. J. Direct and indirect activation of the hippocampus by tubocurarine. J Physiol. 1970 Oct;210(3):697–716. doi: 10.1113/jphysiol.1970.sp009236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann E. Apamin. Pharmacol Ther. 1984;25(2):255–270. doi: 10.1016/0163-7258(84)90046-9. [DOI] [PubMed] [Google Scholar]
- Holzer P., Petsche U. On the mechanism of contraction and desensitization induced by substance P in the intestinal muscle of the guinea-pig. J Physiol. 1983 Sep;342:549–568. doi: 10.1113/jphysiol.1983.sp014868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson D. H., Haylett D. G., Cook N. S. Calcium-activated potassium channels in liver cells. Cell Calcium. 1983 Dec;4(5-6):429–437. doi: 10.1016/0143-4160(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Jenkinson D. H., Morton I. K. The effect of noradrenaline on the permeability of depolarized intestinal smooth muscle to inorganic ions. J Physiol. 1967 Feb;188(3):373–386. doi: 10.1113/jphysiol.1967.sp008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdunski M. Apamin, a neurotoxin specific for one class of Ca2+-dependent K+ channels. Cell Calcium. 1983 Dec;4(5-6):421–428. doi: 10.1016/0143-4160(83)90018-0. [DOI] [PubMed] [Google Scholar]
- Maas A. J., Den Hertog A., Ras R., Van den Akker J. The action of apamin on guinea-pig taenia caeci. Eur J Pharmacol. 1980 Oct 17;67(2-3):265–274. doi: 10.1016/0014-2999(80)90507-5. [DOI] [PubMed] [Google Scholar]
- Maas A. J., Den Hertog A. The effect of apamin on the smooth muscle cells of the guinea-pig taenia coli. Eur J Pharmacol. 1979 Sep 15;58(2):151–156. doi: 10.1016/0014-2999(79)90006-2. [DOI] [PubMed] [Google Scholar]
- North R. A., Williams J. T. On the potassium conductance increased by opioids in rat locus coeruleus neurones. J Physiol. 1985 Jul;364:265–280. doi: 10.1113/jphysiol.1985.sp015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz W., Passow H. Ca2+-activated K+ channels in erythrocytes and excitable cells. Annu Rev Physiol. 1983;45:359–374. doi: 10.1146/annurev.ph.45.030183.002043. [DOI] [PubMed] [Google Scholar]
- Tomita T., Watanabe H. A comparison of the effects of adenosine triphosphate with noradrenaline and with the inhibitory potential of the guinea-pig taenia coli. J Physiol. 1973 May;231(1):167–177. doi: 10.1113/jphysiol.1973.sp010226. [DOI] [PMC free article] [PubMed] [Google Scholar]



