Abstract
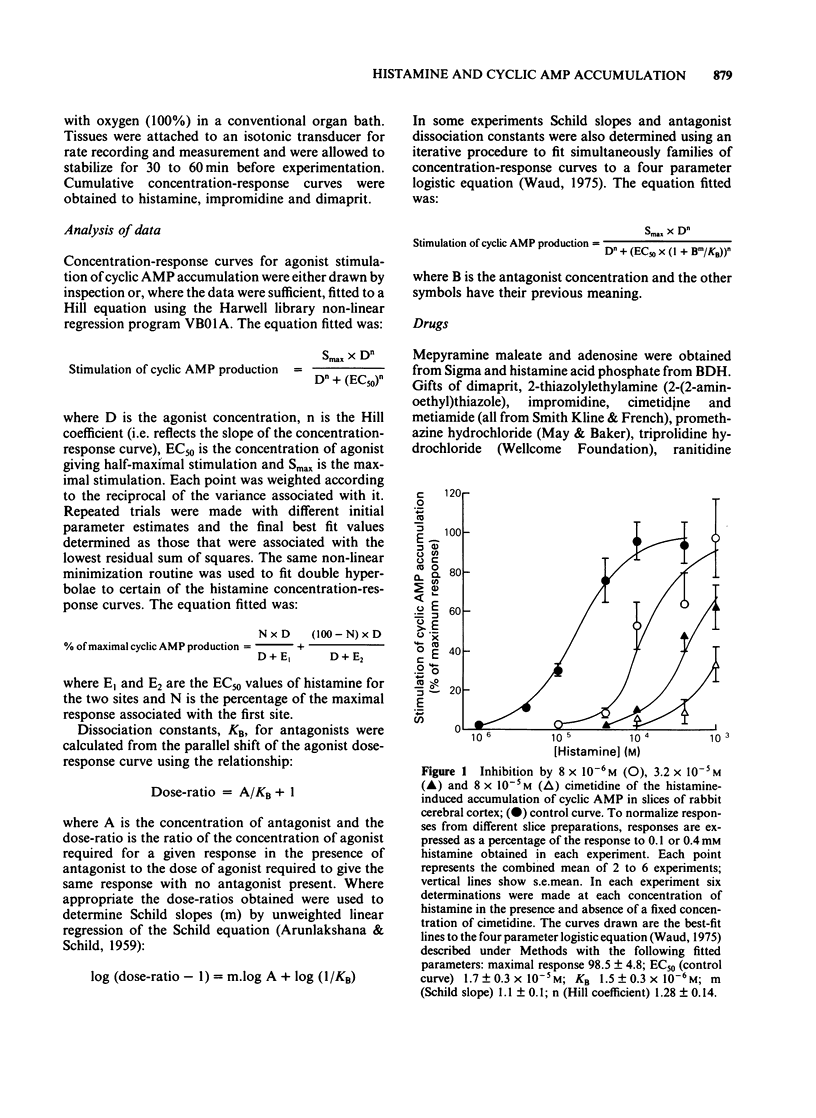
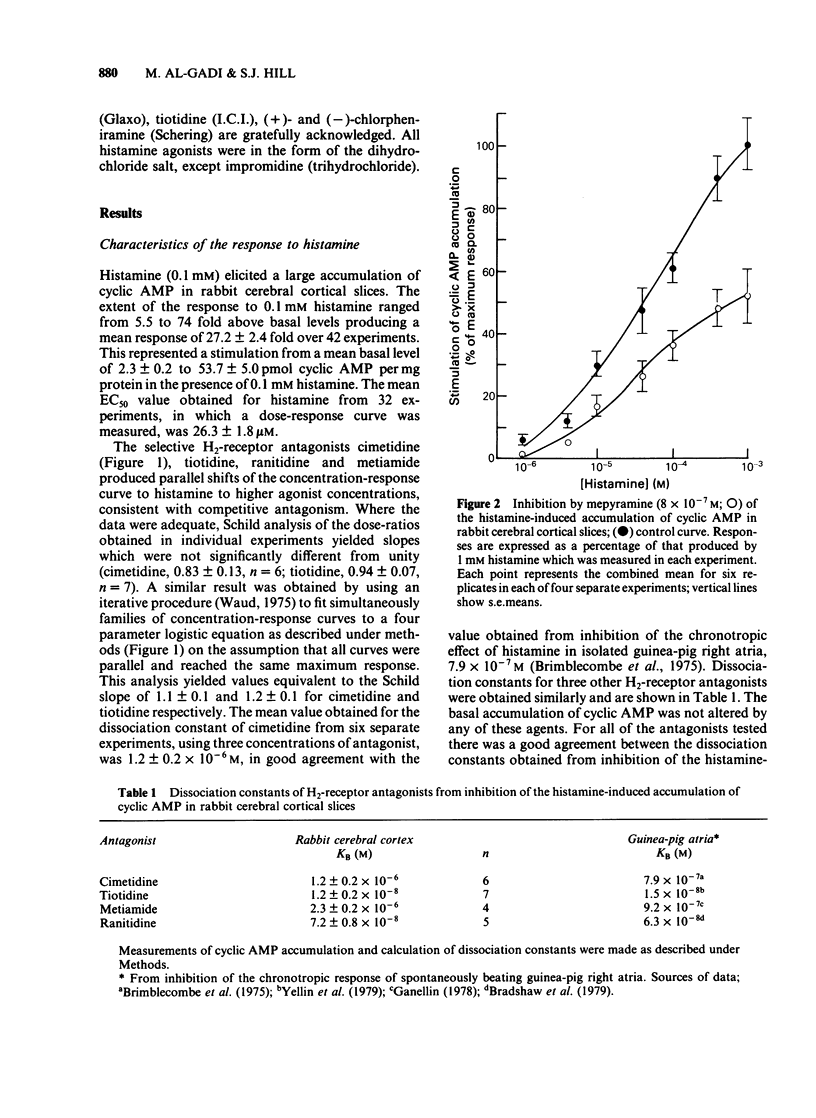
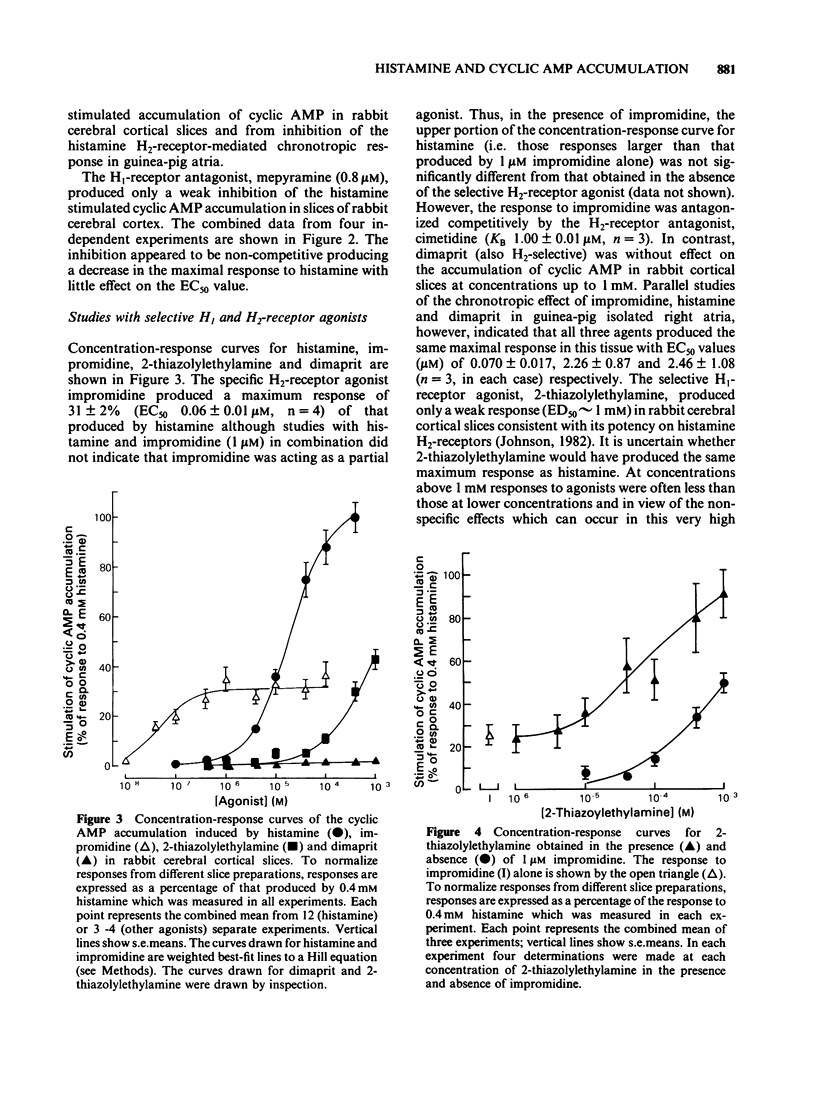
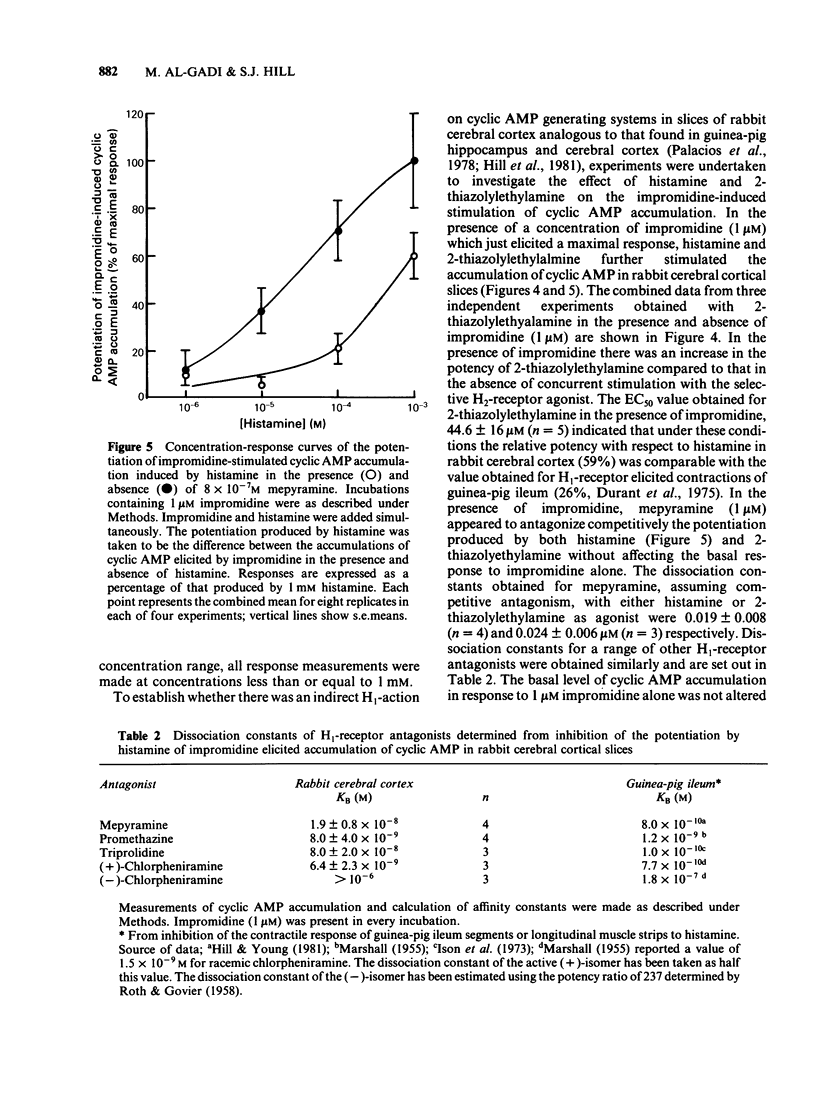
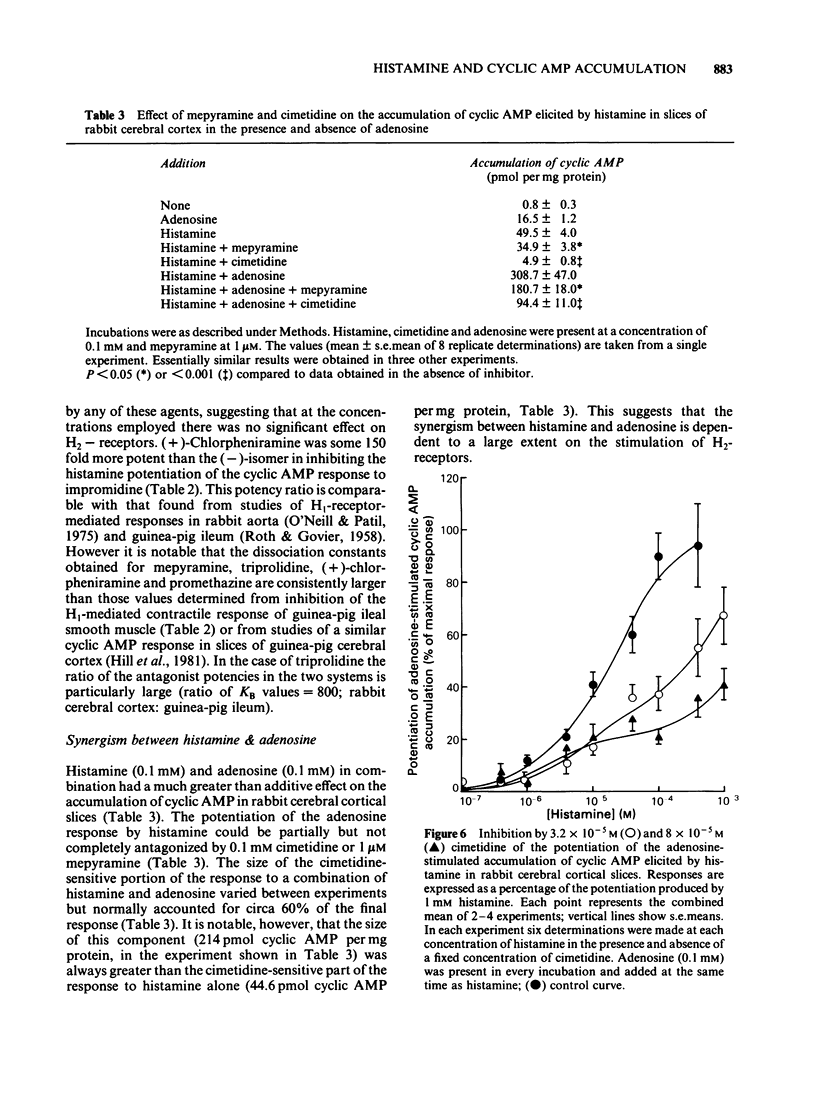
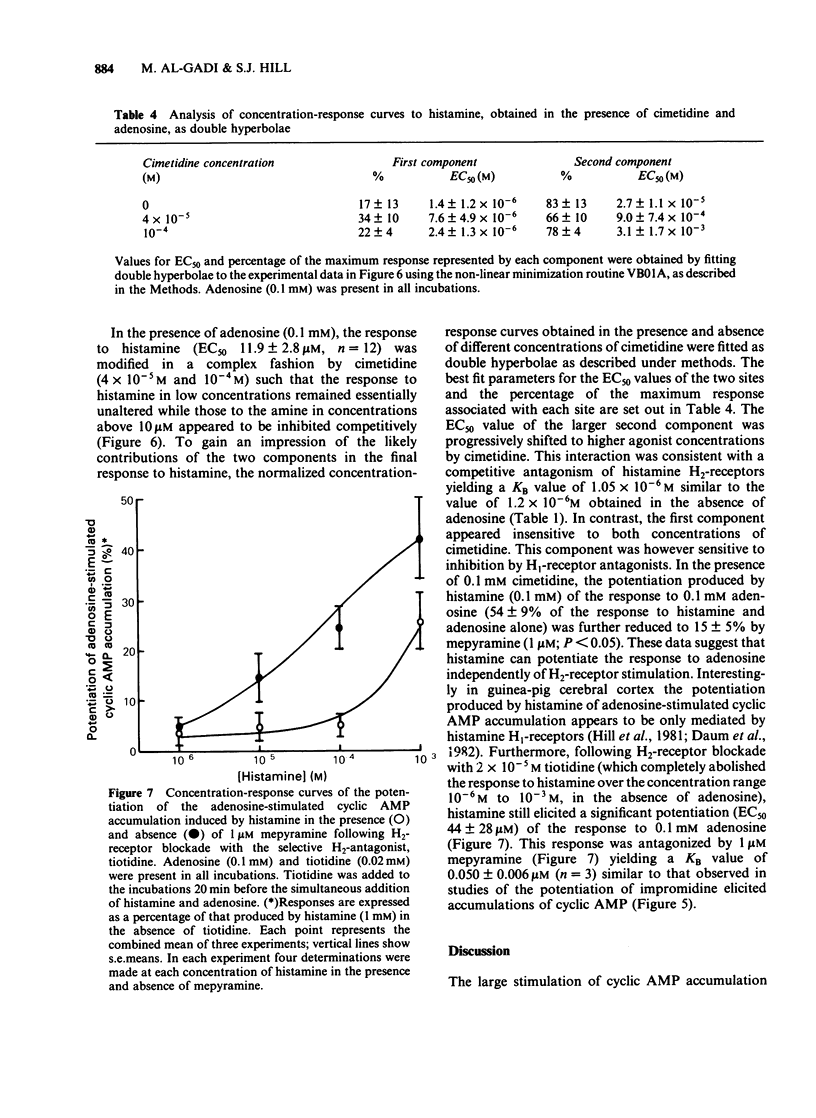
The characteristics of histamine-stimulated adenosine 3':5'-cyclic monophosphate (cyclic AMP) accumulation in slices of rabbit cerebral cortex have been investigated. The selective H2-receptor antagonists, cimetidine, tiotidine, metiamide and ranitidine appeared to antagonize the stimulation of cyclic AMP accumulation elicited by histamine in a competitive manner consistent with an interaction with histamine H2-receptors. The H1-receptor antagonist mepyramine (0.8 microM) produced only a weak inhibition of the response to histamine. The inhibition appeared to be non-competitive producing a decrease in the maximal response with little effect on the EC50 value. The specific H2-receptor agonist, impromidine, produced a maximum response of only 31 +/- 2% of that obtained with histamine. Studies with histamine and impromidine in combination indicated that impromidine was not acting as a partial agonist. 2-Thiazolylethylamine, a selective H1-agonist, produced only a weak response (EC50 approximately 1mM) yielding a relative potency with respect to histamine (= 100) of 2.5. In the presence of a supramaximal concentration of impromidine, histamine and 2-thiazolylethylamine further elevated the response to impromidine. In these conditions the relative potency of 2-thiazolylethylamine was increased to 59 (histamine = 100), a value which was comparable with that reported for H1-receptor-mediated contractions of guinea-pig ileum. The H1-receptor antagonists mepyramine, promethazine, triprolidine and chlorpheniramine competitively antagonized the potentiation of impromidine-stimulated cyclic AMP accumulation elicited by histamine and 2-thiazolylethylamine in rabbit cerebral cortex without affecting the response to impromidine alone. (+)-Chlorpheniramine was some 150 fold more potent than the (-)-isomer in this respect. Histamine and adenosine in combination had a much greater than additive effect on the accumulation of cyclic AMP in rabbit cerebral cortical slices. The potentiation of the adenosine response could be partially but not completely antagonized by either cimetidine or mepyramine. In the presence of H2-receptor blockade with 0.02 mM tiotidine, histamine elicited a significant potentiation (EC50 44 microM) of the response to adenosine. This response was antagonized competitively by mepyramine yielding a KB value of 0.05 microM similar to that obtained from inhibition of the potentiation of impromidine-stimulated accumulation of cyclic AMP (0.02 microM). These results suggest that there are two components in the response to histamine in rabbit cerebral cortical slices.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw J., Brittain R. T., Clitherow J. W., Daly M. J., Jack D., Price B. J., Stables R. Ranitidine (AH 19065): a new potent, selective histamine H2-receptor antagonist [proceedings]. Br J Pharmacol. 1979 Jul;66(3):464P–464P. [PMC free article] [PubMed] [Google Scholar]
- Brown B. L., Ekins R. P., Albano J. D. Saturation assay for cyclic AMP using endogenous binding protein. Adv Cyclic Nucleotide Res. 1972;2:25–40. [PubMed] [Google Scholar]
- Chang R. S., Tran V. T., Snyder S. H. Characteristics of histamine H1-receptors in peripheral tissues labeled with [3H]mepyramine. J Pharmacol Exp Ther. 1979 Jun;209(3):437–442. [PubMed] [Google Scholar]
- Chang R. S., Tran V. T., Snyder S. H. Heterogeneity of histamine H1-receptors: species variations in [3H]mepyramine binding of brain membranes. J Neurochem. 1979 Jun;32(6):1653–1663. doi: 10.1111/j.1471-4159.1979.tb02276.x. [DOI] [PubMed] [Google Scholar]
- Chasin M., Mamrak F., Samaniego G., Hess S. M. Characteristics of the catecholamine and histamine receptor sites mediating accumulation of cyclic adenosine 3',5'-monophosphate in guinea pig brain. J Neurochem. 1973 Dec;21(6):1415–1427. doi: 10.1111/j.1471-4159.1973.tb06026.x. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Padgett W., Nimitkitpaisan Y., Creveling C. R., Cantacuzene D., Kirk K. L. Fluoronorepinephrines: specific agonists for the activation of alpha and beta adrenergic-sensitive cyclic AMP-generating systems in brain slices. J Pharmacol Exp Ther. 1980 Mar;212(3):382–389. [PubMed] [Google Scholar]
- Damerau B., Roesler J., Vogt W. Loss and recovery of sensitivity of guinea-pig isolated ileum to the spasmogenic action of the complement peptide C5adesArg. Br J Pharmacol. 1985 Jan;84(1):47–54. [PMC free article] [PubMed] [Google Scholar]
- Daum P. R., Hill S. J., Young J. M. Histamine H1-agonist potentiation of adenosine-stimulated cyclic AMP accumulation in slices of guinea-pig cerebral cortex: comparison of response and binding parameters. Br J Pharmacol. 1982 Oct;77(2):347–357. doi: 10.1111/j.1476-5381.1982.tb09304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant G. J., Ganellin C. R., Parsons M. E. Chemical differentiation of histamine H1- and H2-receptor agonists. J Med Chem. 1975 Sep;18(9):905–909. doi: 10.1021/jm00243a009. [DOI] [PubMed] [Google Scholar]
- Fleisch J. H., Krzan M. C., Titus E. Alterations in pharmacologic receptor activity by dithiothreitol. Am J Physiol. 1974 Dec;227(6):1243–1248. doi: 10.1152/ajplegacy.1974.227.6.1243. [DOI] [PubMed] [Google Scholar]
- Green J. P., Johnson C. L., Weinstein H., Maayani S. Antagonism of histamine-activated adenylate cyclase in brain by D-lysergic acid diethylamide. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5697–5701. doi: 10.1073/pnas.74.12.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield A. J., Robinson N. R., Hill S. J. The nature of the binding of [3H]mepyramine to homogenates of guinea-pig cerebral cortex at different [3H]ligand concentrations. Biochem Pharmacol. 1983 Aug 15;32(16):2449–2451. doi: 10.1016/0006-2952(83)90690-1. [DOI] [PubMed] [Google Scholar]
- Hegstrand L. R., Kanof P. D., Greengard P. Histamine-sensitive adenylate cyclase in mammalian brain. Nature. 1976 Mar 11;260(5547):163–165. doi: 10.1038/260163a0. [DOI] [PubMed] [Google Scholar]
- Hill S. J., Daum P., Young J. M. Affinities of histamine H1-antagonists in guinea pig brain: similarity of values determined from [3H]mepyramine binding and from inhibition of a functional response. J Neurochem. 1981 Nov;37(5):1357–1360. doi: 10.1111/j.1471-4159.1981.tb04692.x. [DOI] [PubMed] [Google Scholar]
- Hill S. J., Emson P. C., Young J. M. The binding of [3H]mepyramine to histamine H1 receptors in guinea-pig brain. J Neurochem. 1978 Oct;31(4):997–1004. doi: 10.1111/j.1471-4159.1978.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Hill S. J., Young J. M. Characterization of [3H]mepyramine binding to the longitudinal muscle of guinea pig small intestine. Mol Pharmacol. 1981 May;19(3):379–387. [PubMed] [Google Scholar]
- Hill S. J., Young J. M. Histamine H1-receptors in the brain of the guinea-pig and the rat: differences in ligand binding properties and regional distribution. Br J Pharmacol. 1980 Apr;68(4):687–696. doi: 10.1111/j.1476-5381.1980.tb10861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison R. R., Franks F. M., Soh K. S. The binding of conformationally restricted antihistamines to histamine receptors. J Pharm Pharmacol. 1973 Nov;25(11):887–894. doi: 10.1111/j.2042-7158.1973.tb09968.x. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Rall T. W. Studies on adenosine 3',5'-phosphate in rabbit cerebral cortex. Mol Pharmacol. 1968 Jul;4(4):379–388. [PubMed] [Google Scholar]
- Kakiuchi S., Rall T. W. The influence of chemical agents on the accumulation of adenosine 3',5'-Phosphate in slices of rabbit cerebellum. Mol Pharmacol. 1968 Jul;4(4):367–378. [PubMed] [Google Scholar]
- Kenakin T. P., Beek D. Is prenalterol (H133/80) really a selective beta 1 adrenoceptor agonist? Tissue selectivity resulting from differences in stimulus-response relationships. J Pharmacol Exp Ther. 1980 May;213(2):406–413. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARSHALL P. B. Some chemical and physical properties associated with histamine antagonism. Br J Pharmacol Chemother. 1955 Sep;10(3):270–278. doi: 10.1111/j.1476-5381.1955.tb00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill P. J., Patil P. N. Stereoisomers of an antihistamine and the pharmacologic receptors of rabbit aorta. Pharmacol Res Commun. 1975 Jun;7(3):273–279. doi: 10.1016/0031-6989(75)90026-0. [DOI] [PubMed] [Google Scholar]
- Palacios J. M., Garbarg M., Barbin G., Schwartz J. C. Pharmacological characterization of histamine receptors mediating the stimulation of cyclic AMP accumulation in slices from guinea-pig hippocampus. Mol Pharmacol. 1978 Nov;14(6):971–982. [PubMed] [Google Scholar]
- Palmer G. C., Schmidt M. J., Robinson G. A. Development and characteristics of the histamine-induced accumulation of cyclic AMP in the rabbit cerebral cortex. J Neurochem. 1972 Oct;19(10):2251–2256. doi: 10.1111/j.1471-4159.1972.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Parsons M. E., Blakemore R. C., Durant G. J., Ganellin C. R., Rasmussen A. C. Proceedings: 3-(4(5)-imidazolyl) propylguanidine (SK&F 91486) - a partial agonist at histamine H2-receptors. Agents Actions. 1975 Dec;5(5):464–464. doi: 10.1007/BF01972676. [DOI] [PubMed] [Google Scholar]
- ROTH F. E., GOVIER W. M. Comparative pharmacology of chlorpheniramine (Chlor-Trimeton) and its optical isomers. J Pharmacol Exp Ther. 1958 Dec;124(4):347–349. [PubMed] [Google Scholar]
- Schwabe U., Ohga Y., Daly J. W. The role of calcium in the regulation of cyclic nucleotide levels in brain slices of rat and guinea pig. Naunyn Schmiedebergs Arch Pharmacol. 1978 Apr;302(2):141–151. doi: 10.1007/BF00517981. [DOI] [PubMed] [Google Scholar]
- Spiker M. D., Palmer G. C., Manian A. A. Action of neuroleptic agents on histamine-sensitive adenylate cyclase in rabbit cerebral cortex. Brain Res. 1976 Mar 12;104(2):401–406. doi: 10.1016/0006-8993(76)90640-5. [DOI] [PubMed] [Google Scholar]
- Yellin T. O., Buck S. H., Gilman D. J., Jones D. F., Wardleworth J. M. ICI 125,211: a new gastric antisecretory agent acting on histamine H2-receptors. Life Sci. 1979 Dec 3;25(23):2001–2009. doi: 10.1016/0024-3205(79)90604-0. [DOI] [PubMed] [Google Scholar]


