Abstract
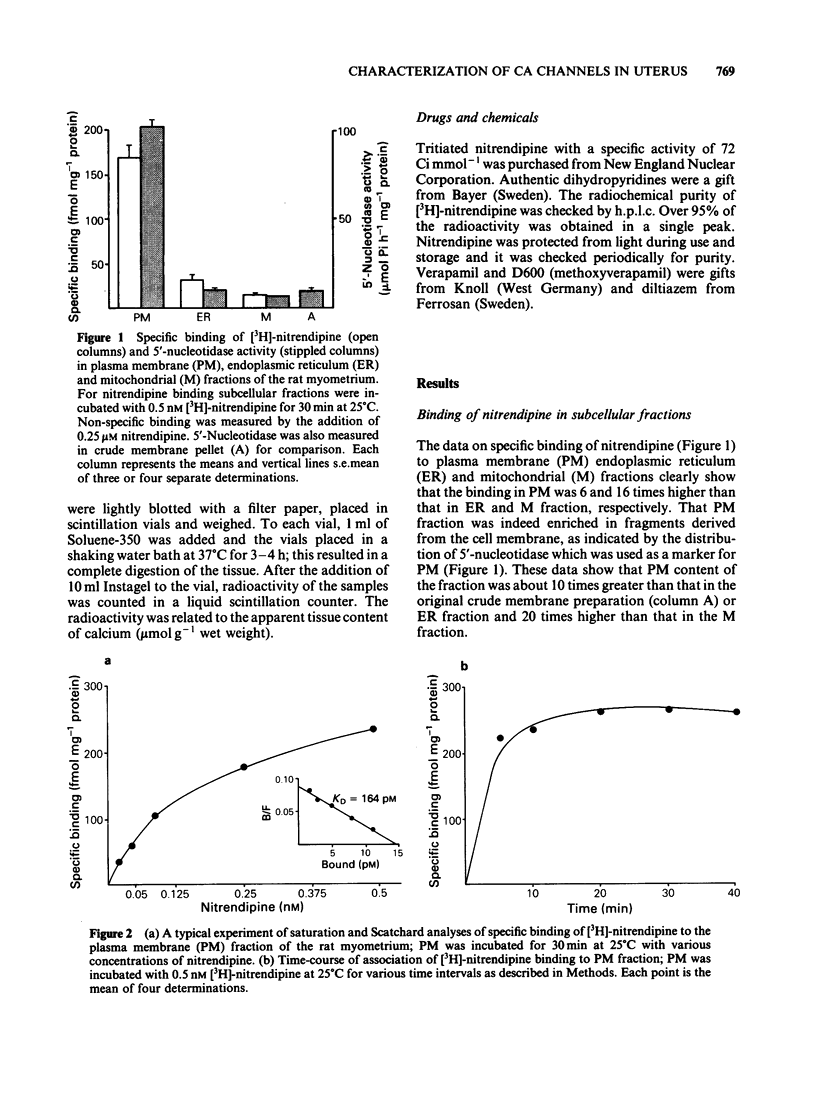
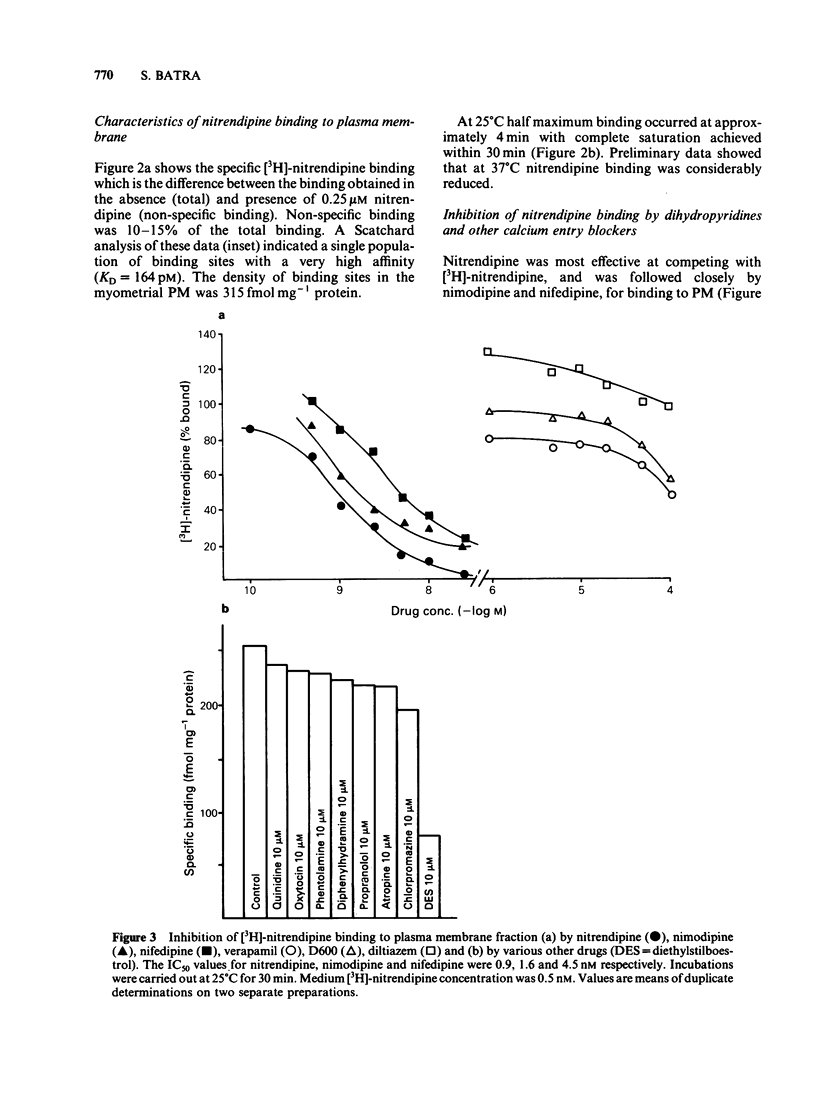
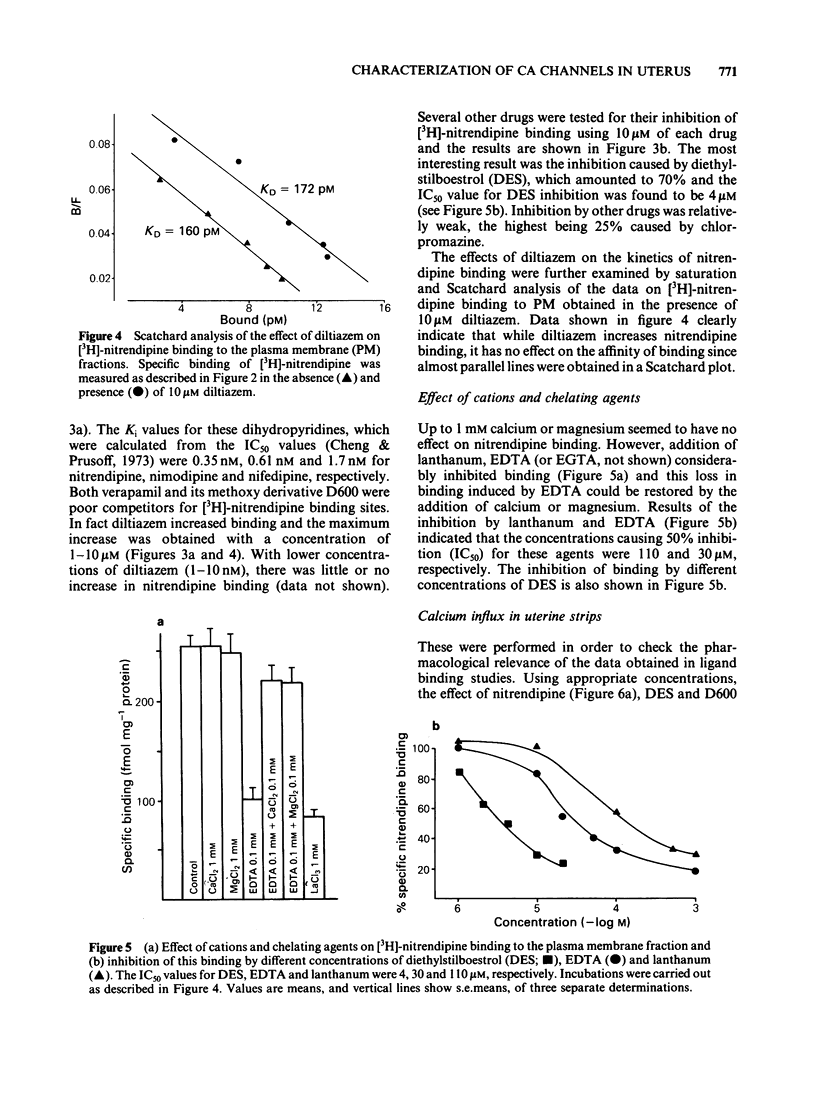
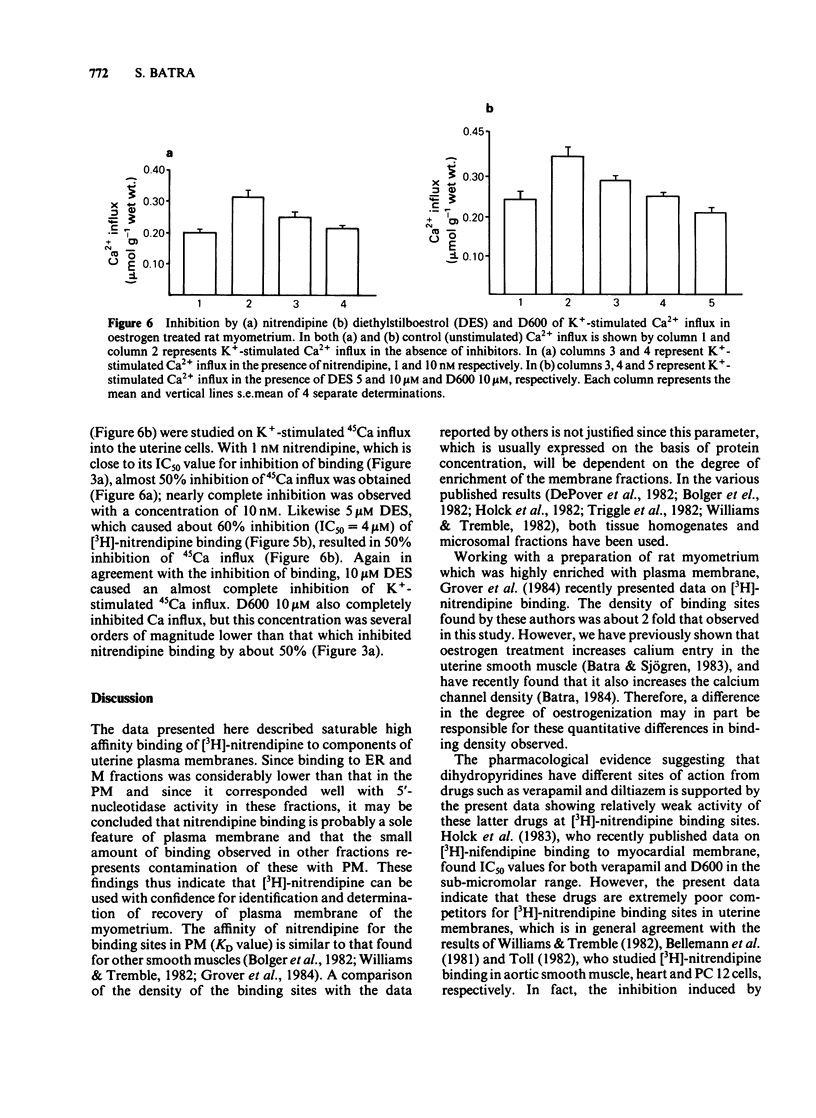
Specific, high affinity (KD = 164 pM) binding of the Ca channel inhibitor [3H]-nitrendipine was identified in plasma membrane-enriched fractions from the rat myometrium. Although dihydropyridines effectively competed for [3H]-nitrendipine binding sites, both verapamil and D600 were poor competitors. Diltiazem (10 microM) increased [3H]-nitrendipine binding by about 40%, but had no effect on binding affinity. Among several other drugs tested, diethylstilboestrol (DES) caused a considerable inhibition of binding, with an IC50 value of 4 microM. Both La3+ and EDTA (or EGTA) inhibited binding. The inhibition by the latter could be overcome by the addition of Ca2+ or Mg2+. A clear relationship was found between [3H]-nitrendipine binding and 5-nucleotidase activity in the various subcellular fractions. Data on K+-stimulated Ca2+ influx in the intact uterine strips showed a good agreement between the inhibition by both nitrendipine and DES of stimulated Ca influx and their inhibitory effect on [3H]-nitrendipine binding to plasma membrane. This type of correlation was lacking in the case of D600. These results suggest that Ca channels in the myometrial membrane possess multiple sites at which different drugs can act to block these channels.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batra S., Bengtsson B. Effects of diethylstilboestrol and ovarian steroids on the contractile responses and calcium movements in rat uterine smooth muscle. J Physiol. 1978 Mar;276:329–342. doi: 10.1113/jphysiol.1978.sp012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra S., Sjöberg N. O., Thorbert G. Estrogen and progesterone interactions in the rabbit uterus in vivo after steroid administration. Endocrinology. 1978 Jan;102(1):268–272. doi: 10.1210/endo-102-1-268. [DOI] [PubMed] [Google Scholar]
- Batra S., Sjögren C. Effect of estrogen treatment on calcium uptake by the rat uterine smooth muscle. Life Sci. 1983 Jan 24;32(4):315–319. doi: 10.1016/0024-3205(83)90076-0. [DOI] [PubMed] [Google Scholar]
- Batra S. Uptake and energy-dependent extrusion of calcium in the rat uterus. Acta Physiol Scand. 1982 Mar;114(3):447–452. doi: 10.1111/j.1748-1716.1982.tb07008.x. [DOI] [PubMed] [Google Scholar]
- Bellemann P., Ferry D., Lübbecke F., Glossman H. [3H]-Nitrendipine, a potent calcium antagonist, binds with high affinity to cardiac membranes. Arzneimittelforschung. 1981;31(12):2064–2067. [PubMed] [Google Scholar]
- Boles R. G., Yamamura H. I., Schoemaker H., Roeske W. R. Temperature-dependent modulation of [3H]nitrendipine binding by the calcium channel antagonists verapamil and diltiazem in rat brain synaptosomes. J Pharmacol Exp Ther. 1984 May;229(2):333–339. [PubMed] [Google Scholar]
- Bolger G. T., Gengo P. J., Luchowski E. M., Siegel H., Triggle D. J., Janis R. A. High affinity binding of a calcium channel antagonist to smooth and cardiac muscle. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1604–1609. doi: 10.1016/0006-291x(82)91436-x. [DOI] [PubMed] [Google Scholar]
- Bolger G. T., Gengo P., Klockowski R., Luchowski E., Siegel H., Janis R. A., Triggle A. M., Triggle D. J. Characterization of binding of the Ca++ channel antagonist, [3H]nitrendipine, to guinea-pig ileal smooth muscle. J Pharmacol Exp Ther. 1983 May;225(2):291–309. [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- DePover A., Matlib M. A., Lee S. W., Dubé G. P., Grupp I. L., Grupp G., Schwartz A. Specific binding of [3H]nitrendipine to membranes from coronary arteries and heart in relation to pharmacological effects. Paradoxical stimulation by diltiazem. Biochem Biophys Res Commun. 1982 Sep 16;108(1):110–117. doi: 10.1016/0006-291x(82)91838-1. [DOI] [PubMed] [Google Scholar]
- Ferry D. R., Goll A., Glossmann H. Differential labelling of putative skeletal muscle calcium channels by [3H]-nifedipine, [3H]-nitrendipine, [3H]-nimodipine and [3H]-PN 200 110. Naunyn Schmiedebergs Arch Pharmacol. 1983 Jul;323(3):276–277. doi: 10.1007/BF00497674. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R., Lübbecke F., Mewes R., Hofmann F. Identification of voltage operated calcium channels by binding studies: differentiation of subclasses of calcium antagonist drugs with 3H-nimodipine radioligand binding. J Recept Res. 1983;3(1-2):177–190. doi: 10.3109/10799898309041932. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Linn T., Rombusch M., Ferry D. R. Temperature-dependent regulation of d-cis-[3H]diltiazem binding to Ca2+ channels by 1,4-dihydropyridine channel agonists and antagonists. FEBS Lett. 1983 Aug 22;160(1-2):226–232. doi: 10.1016/0014-5793(83)80972-7. [DOI] [PubMed] [Google Scholar]
- Godfraind T. Proceedings: The action of cinnarizine and of phentolamine on the noradrenaline-dependent calcium influx in vascular smooth muscle. Br J Pharmacol. 1974 Sep;52(1):120P–120P. [PMC free article] [PubMed] [Google Scholar]
- Grover A. K., Kwan C. Y., Luchowski E., Daniel E. E., Triggle D. J. Subcellular distribution of [3H]nitrendipine binding in smooth muscle. J Biol Chem. 1984 Feb 25;259(4):2223–2226. [PubMed] [Google Scholar]
- Holck M., Thorens S., Haeusler G. Characterization of [3H]nifedipine binding sites in rabbit myocardium. Eur J Pharmacol. 1982 Dec 3;85(3-4):305–315. doi: 10.1016/0014-2999(82)90217-5. [DOI] [PubMed] [Google Scholar]
- Holck M., Thorens S., Haeusler G. Does [3H]nifedipine label the calcium channel in rabbit myocardium? J Recept Res. 1983;3(1-2):191–198. doi: 10.3109/10799898309041933. [DOI] [PubMed] [Google Scholar]
- Jaqua-Stewart M. J., Read W. O., Steffen R. P. Isolation of pure myocardial subcellular organelles. Anal Biochem. 1979 Jul 15;96(2):293–297. doi: 10.1016/0003-2697(79)90584-0. [DOI] [PubMed] [Google Scholar]
- Murphy K. M., Gould R. J., Largent B. L., Snyder S. H. A unitary mechanism of calcium antagonist drug action. Proc Natl Acad Sci U S A. 1983 Feb;80(3):860–864. doi: 10.1073/pnas.80.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanui H. Measurement of inorganic orthophosphate in biological materials: extraction properties of butyl acetate. Anal Biochem. 1974 Aug;60(2):489–504. doi: 10.1016/0003-2697(74)90259-0. [DOI] [PubMed] [Google Scholar]
- Toll L. Calcium antagonists High-affinity binding and inhibition of calcium transport in a clonal cell line. J Biol Chem. 1982 Nov 25;257(22):13189–13192. [PubMed] [Google Scholar]
- Triggle C. R., Agrawal D. K., Bolger G. T., Daniel E. E., Kwan C. Y., Luchowski E. M., Triggle D. J. Calcium-channel antagonist binding to isolated vascular smooth muscle membranes. Can J Physiol Pharmacol. 1982 Dec;60(12):1738–1741. doi: 10.1139/y82-257. [DOI] [PubMed] [Google Scholar]
- Van Breemen C., Farinas B. R., Gerba P., McNaughton E. D. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res. 1972 Jan;30(1):44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Tremble P. Binding of a calcium antagonist, [3H]nitrendipine, to high affinity sites in bovine aortic smooth muscle and canine cardiac membranes. J Clin Invest. 1982 Jul;70(1):209–212. doi: 10.1172/JCI110596. [DOI] [PMC free article] [PubMed] [Google Scholar]


