Abstract
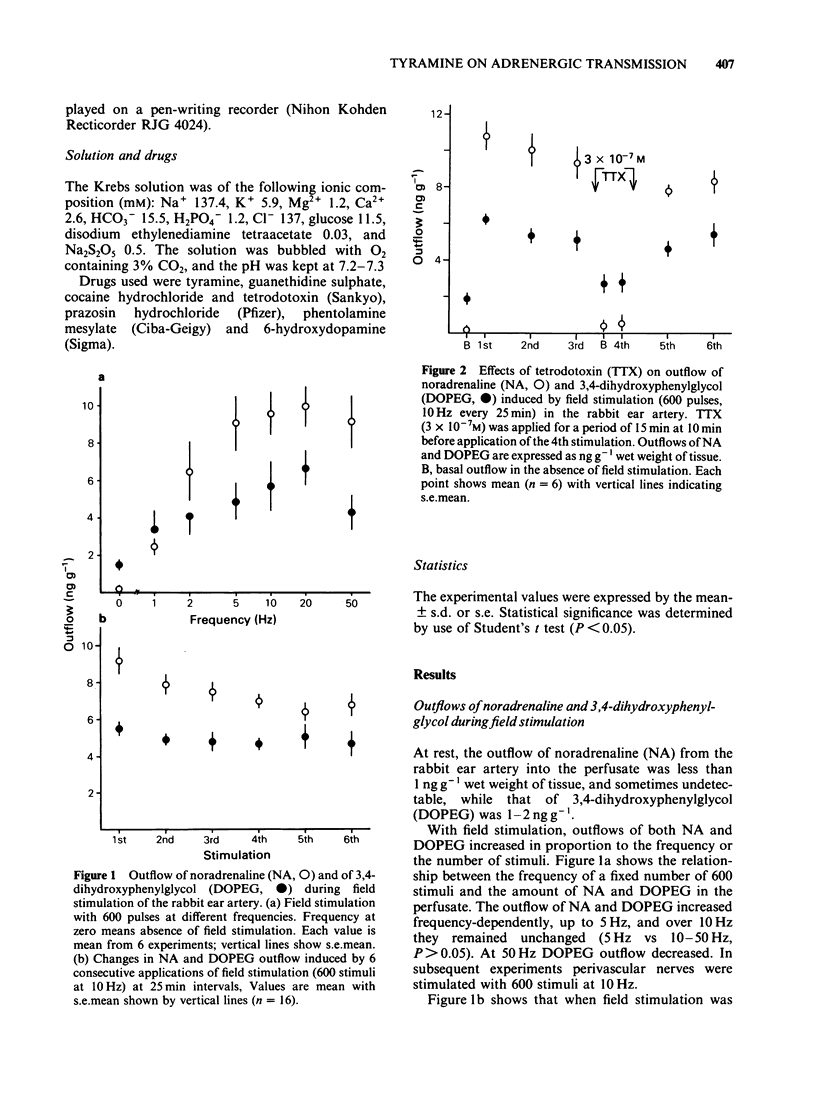
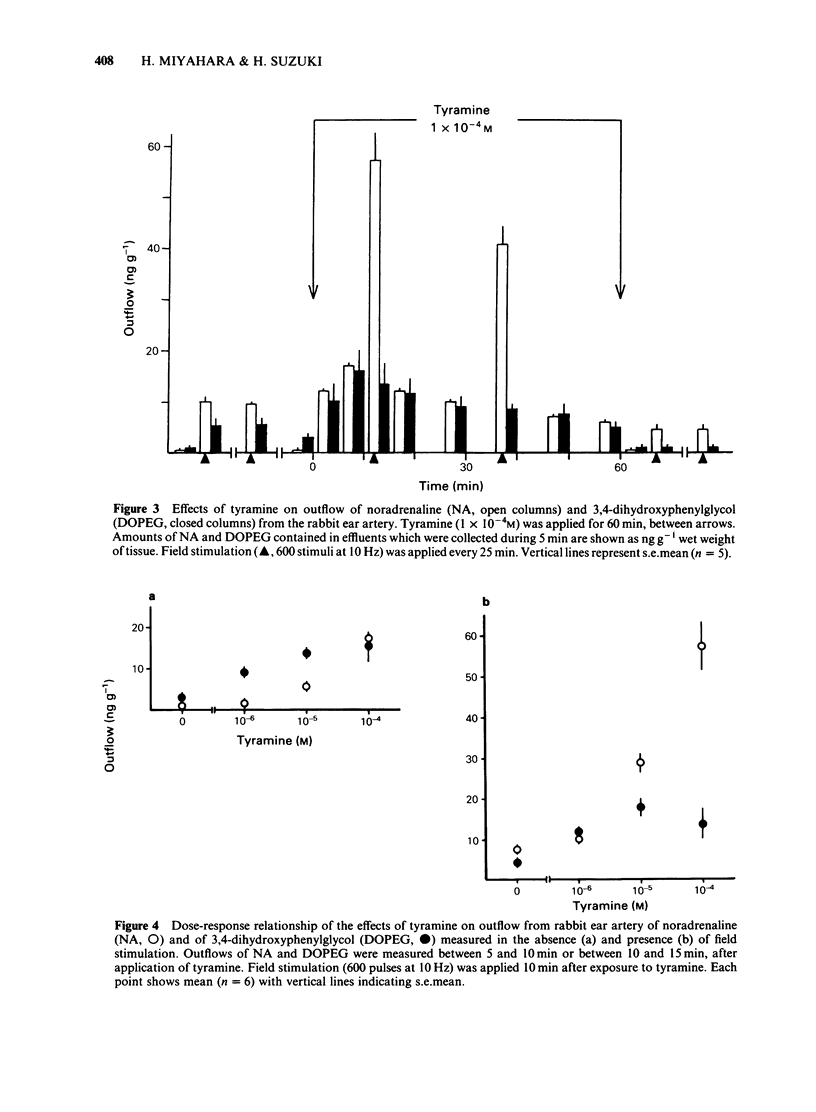
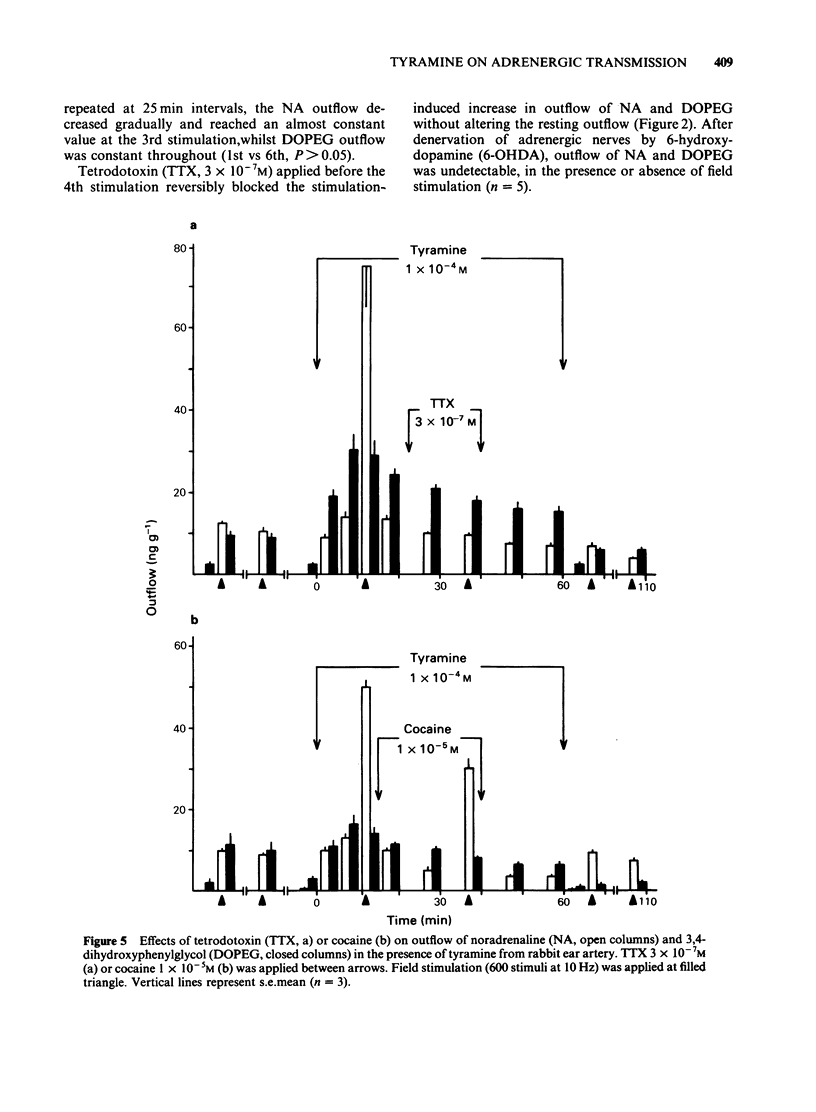
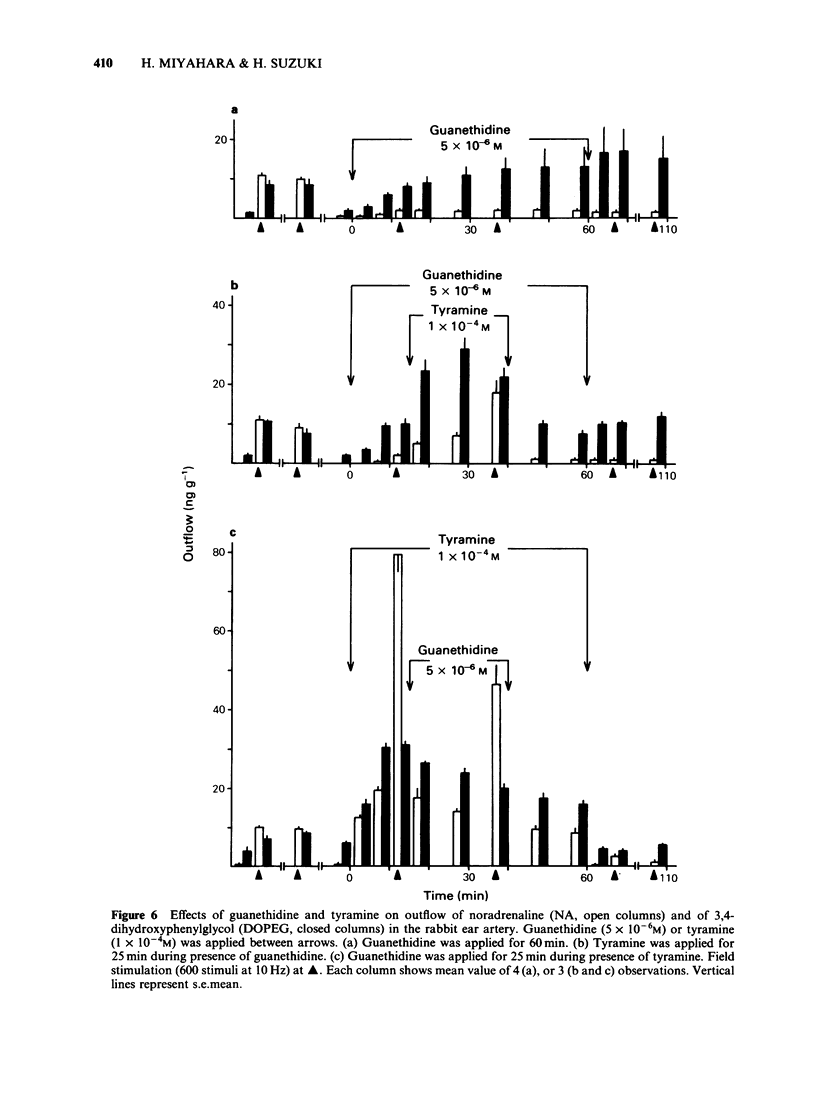
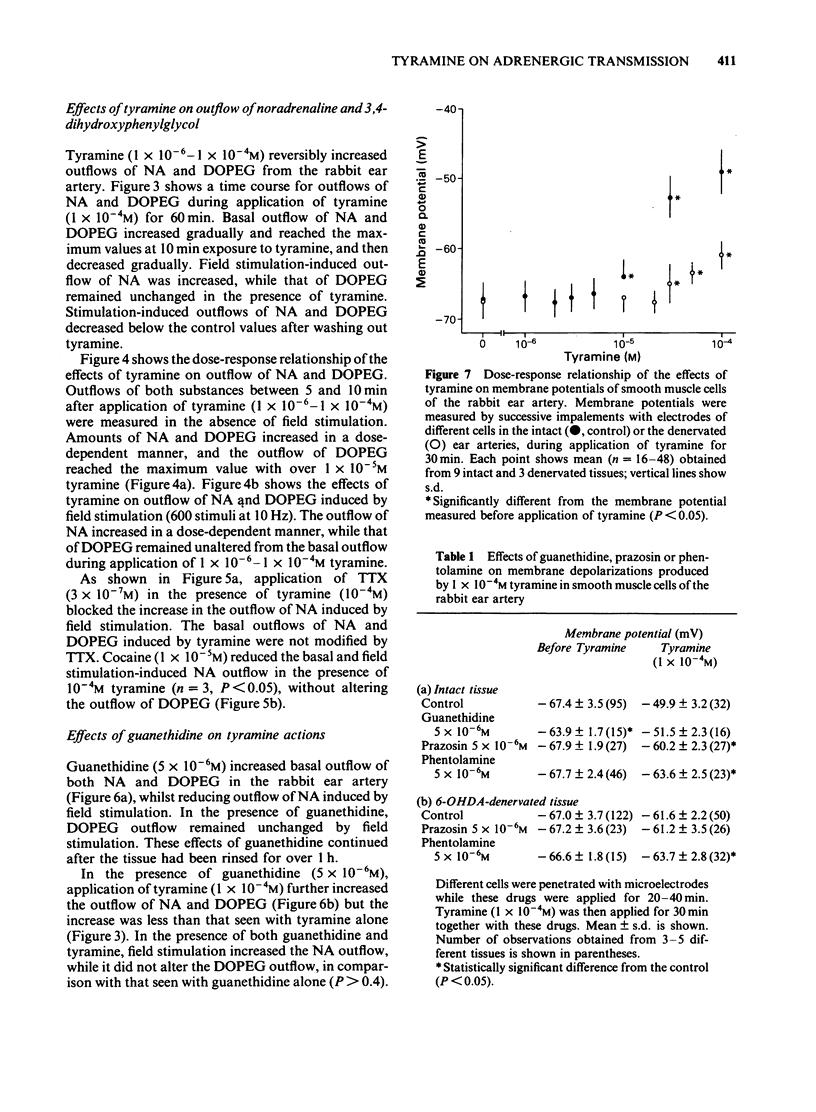
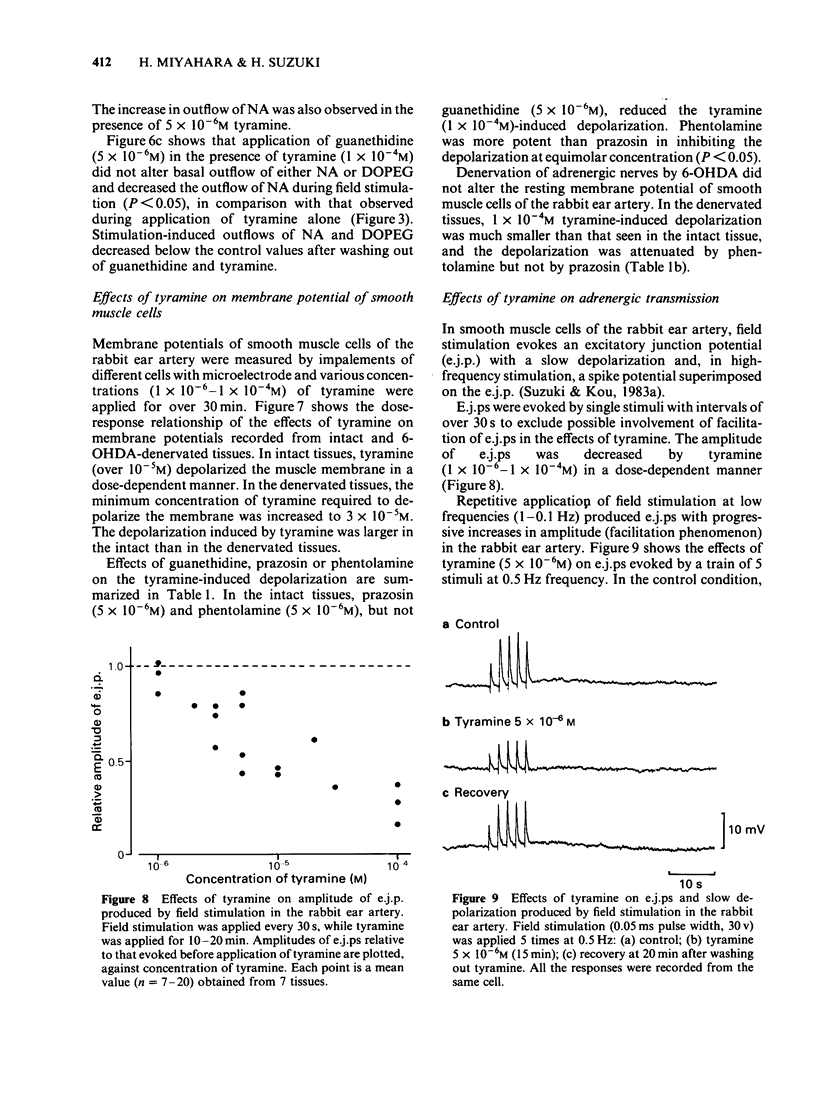
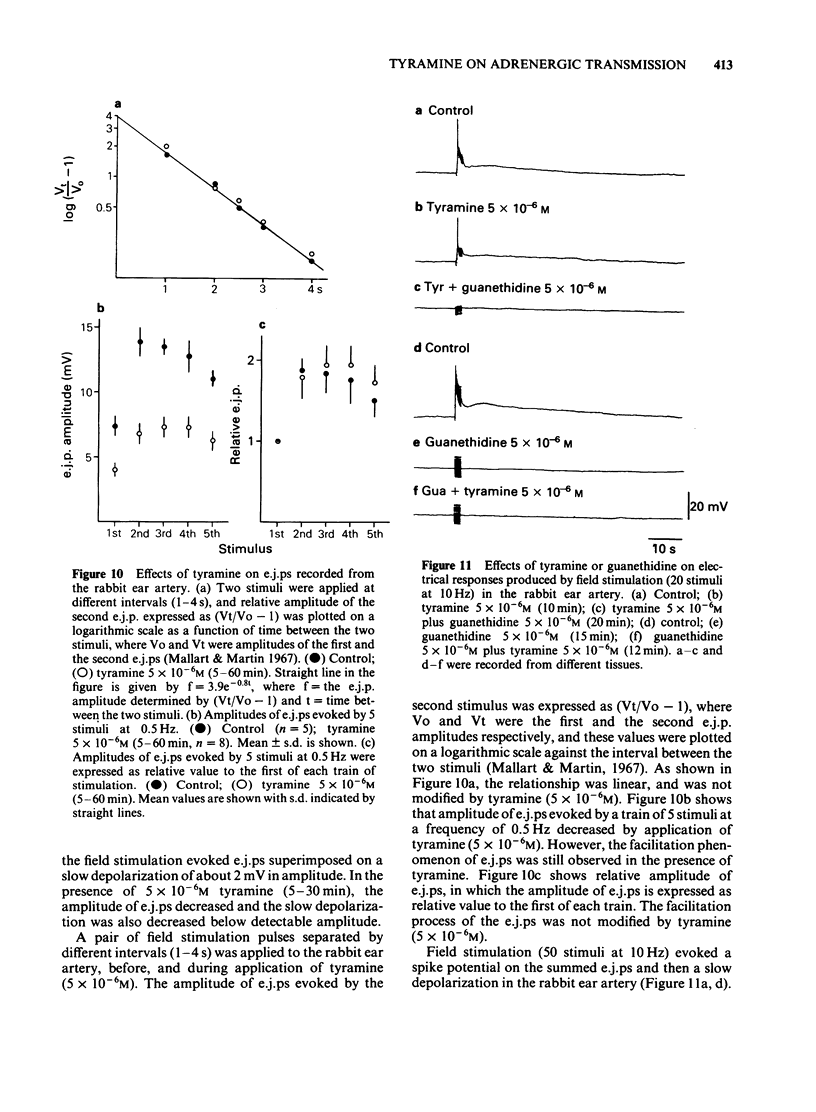
In the perfused rabbit ear artery the basal outflows of noradrenaline (NA) and 3,4-dihydroxyphenylglycol (DOPEG) were less than 1 ng g-1 and 1-2 ng g-1 wet weight of tissue respectively. Field stimulation increased outflows of NA and DOPEG in a frequency-dependent manner, and they reached the maximum value at frequencies over 5 Hz. Tyramine (1 X 10(-6) -1 X 10(-4) M) increased basal outflow of NA and DOPEG, in a dose-dependent manner. This effect was not blocked by tetrodotoxin (TTX, 3 X 10(-7) M), but was prevented by pretreatment with 6-hydroxydopamine (6-OHDA). Tyramine increased the field stimulation-induced outflow of NA but not that of DOPEG in a dose-dependent manner. Cocaine (1 X 10(-5) M) reduced the increased outflow of NA induced by tyramine at rest and during field stimulation, without modifying DOPEG-outflow. Guanethidine (5 X 10(-6) M), increased outflows of NA and DOPEG at rest, and reduced the NA outflow induced by field stimulation. Pretreatment with guanethidine (5 X 10(-6) M) did not block the action of tyramine on NA and DOPEG basal outflows. Additional application of guanethidine during the presence of tyramine did reduce the outflow of NA induced by field stimulation, but did not modify the outflow of NA and DOPEG at rest. Tyramine at concentrations over 1 X 10(-5) M depolarized the smooth muscle membrane of the rabbit ear artery. After chemical denervation with 6-hydroxydopamine (6-OHDA) the depolarizing action of tyramine was reduced. Tyramine-induced depolarization was attenuated by prazosin (5 X 10(-6) M) or phentolamine (5 X 10(-6) M), but not by guanethidine (5 X 10(-6) M). In 6-OHDA-denervated tissues, tyramine-induced depolarization was attenuated by phentolamine but not by prazosin. Field stimulation evoked excitatory junction potential (e.j.p.), slow depolarization and spike potential in the rabbit ear artery. Tyramine reduced, while guanethidine blocked these electrical responses. Tyramine did not alter the facilitation process of e.j.ps. In tissues pretreated with guanethidine, tyramine evoked either no electrical response or a slow depolarization during field stimulation. The slow depolarization was blocked by prazosin. Tyramine reduced the NA content of tissues in a dose-dependent manner (by 31% at 10(-4) M). Guanethidine (5 X 10(-6) M) reduced the NA content by 20%.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aprigliano O., Hermsmeyer K. In vitro denervation of the portal vein and caudal artery of the rat. J Pharmacol Exp Ther. 1976 Sep;198(3):568–577. [PubMed] [Google Scholar]
- De la Lande I. S., Waterson J. G. A comparison of the pharmacology of the isolated rabbit ear and its central artery. Aust J Exp Biol Med Sci. 1968 Dec;46(6):739–745. doi: 10.1038/icb.1968.180. [DOI] [PubMed] [Google Scholar]
- Graefe K. H., Henseling M. Neuronal and extraneuronal uptake and metabolism of catecholamines. Gen Pharmacol. 1983;14(1):27–33. doi: 10.1016/0306-3623(83)90058-7. [DOI] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. An electrophysiological analysis of the effects of noradrenaline and alpha-receptor antagonists on neuromuscular transmission in mammalian muscular arteries. Br J Pharmacol. 1980;71(2):651–661. doi: 10.1111/j.1476-5381.1980.tb10986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara M., Casteels R. Effects of Ca-antagonists on neuromuscular transmission in the rabbit ear artery. Pflugers Arch. 1983 Jan;396(1):1–7. doi: 10.1007/BF00584690. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Makita Y. Modulation of noradrenergic transmission in the guinea-pig mesenteric artery: an electrophysiological study. J Physiol. 1983 Feb;335:609–627. doi: 10.1113/jphysiol.1983.sp014554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. Sixth gaddum memorial lecture, National Institute for Medical Research, Mill Hill, January 1977. Presynaptic receptors and their role in the regulation of transmitter release. Br J Pharmacol. 1977 Aug;60(4):481–497. doi: 10.1111/j.1476-5381.1977.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima S., Miyahara H., Suzuki H. Transmitter release modulated by alpha-adrenoceptor antagonists in the rabbit mesenteric artery: a comparison between noradrenaline outflow and electrical activity. Br J Pharmacol. 1984 Oct;83(2):537–547. doi: 10.1111/j.1476-5381.1984.tb16518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. R., Oates J. A. Guanethidine and related agents. I. Mechanism of the selective blockade of adrenergic neurons and its antagonism by drugs. J Pharmacol Exp Ther. 1970 Mar;172(1):100–107. [PubMed] [Google Scholar]
- Oishi R., Mishima S., Kuriyama H. Determination of norepinephrine and its metabolites released from rat vas deferens using high-performance liquid chromatography with electrochemical detection. Life Sci. 1983 Feb 28;32(9):933–940. doi: 10.1016/0024-3205(83)90922-0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Takimoto G. S., Cho A. K. Compartmental analysis of tyramine-induced norepinephrine depletion. Pharmacology. 1981;22(4):235–242. doi: 10.1159/000137495. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Suzuki H. Adrenergic transmission in the dog mesenteric vein and its modulation by alpha-adrenoceptor antagonists. Br J Pharmacol. 1984 Mar;81(3):479–489. doi: 10.1111/j.1476-5381.1984.tb10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Kou K. Direct and indirect effects of histamine on the smooth muscle cells of the guinea-pig main pulmonary artery. Pflugers Arch. 1983 Sep;399(1):46–53. doi: 10.1007/BF00652521. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kou K. Electrical components contributing to the nerve-mediated contractions in the smooth muscles of the rabbit ear artery. Jpn J Physiol. 1983;33(5):743–756. doi: 10.2170/jjphysiol.33.743. [DOI] [PubMed] [Google Scholar]
- Toda N., Hayashi S., Hattori K. Analysis of the effect of tyramine and norepinephrine in isolated canine cerebral and mesenteric arteries. J Pharmacol Exp Ther. 1978 May;205(2):382–391. [PubMed] [Google Scholar]
- Vanhoutte P. M., Verbeuren T. J., Webb R. C. Local modulation of adrenergic neuroeffector interaction in the blood vessel well. Physiol Rev. 1981 Jan;61(1):151–247. doi: 10.1152/physrev.1981.61.1.151. [DOI] [PubMed] [Google Scholar]


