Abstract
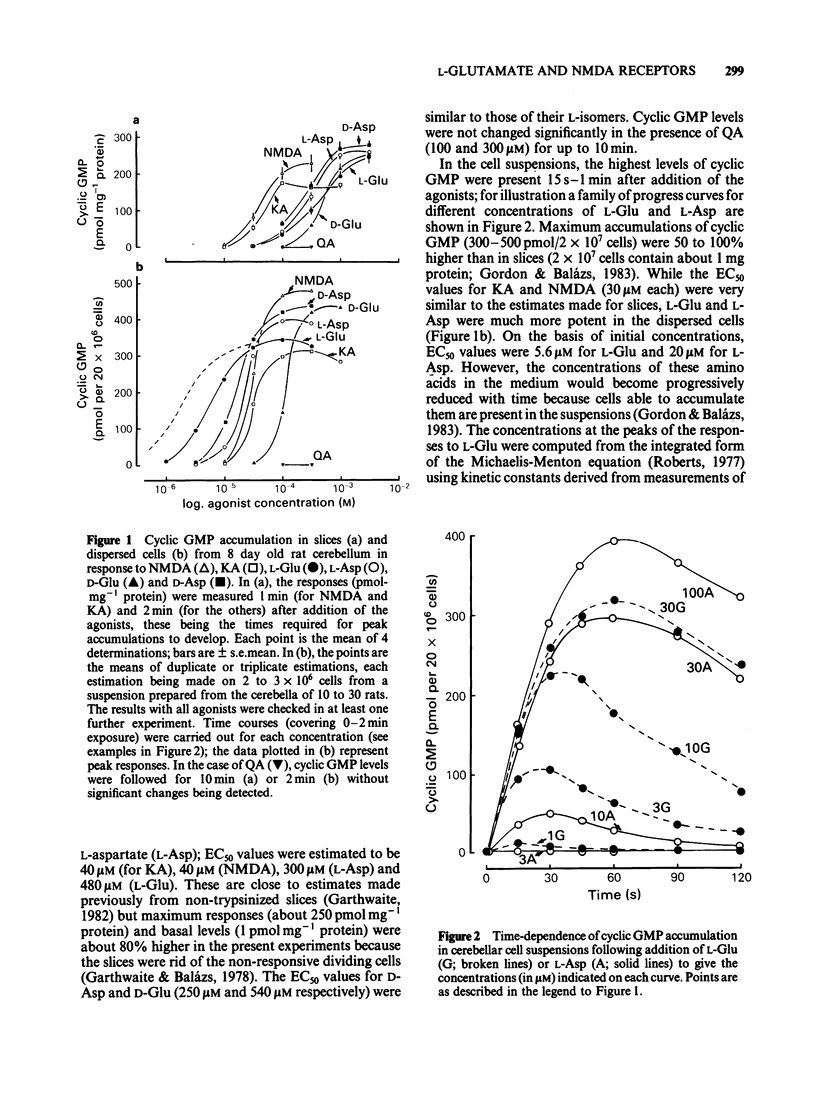
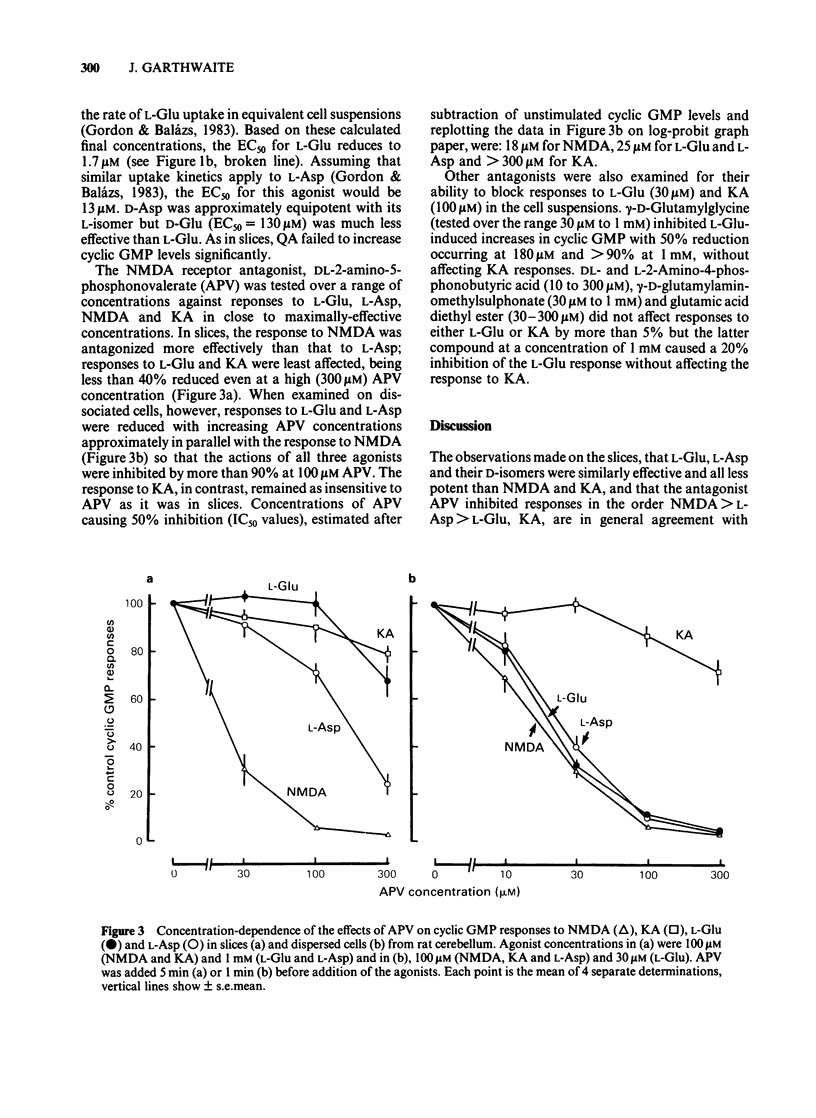
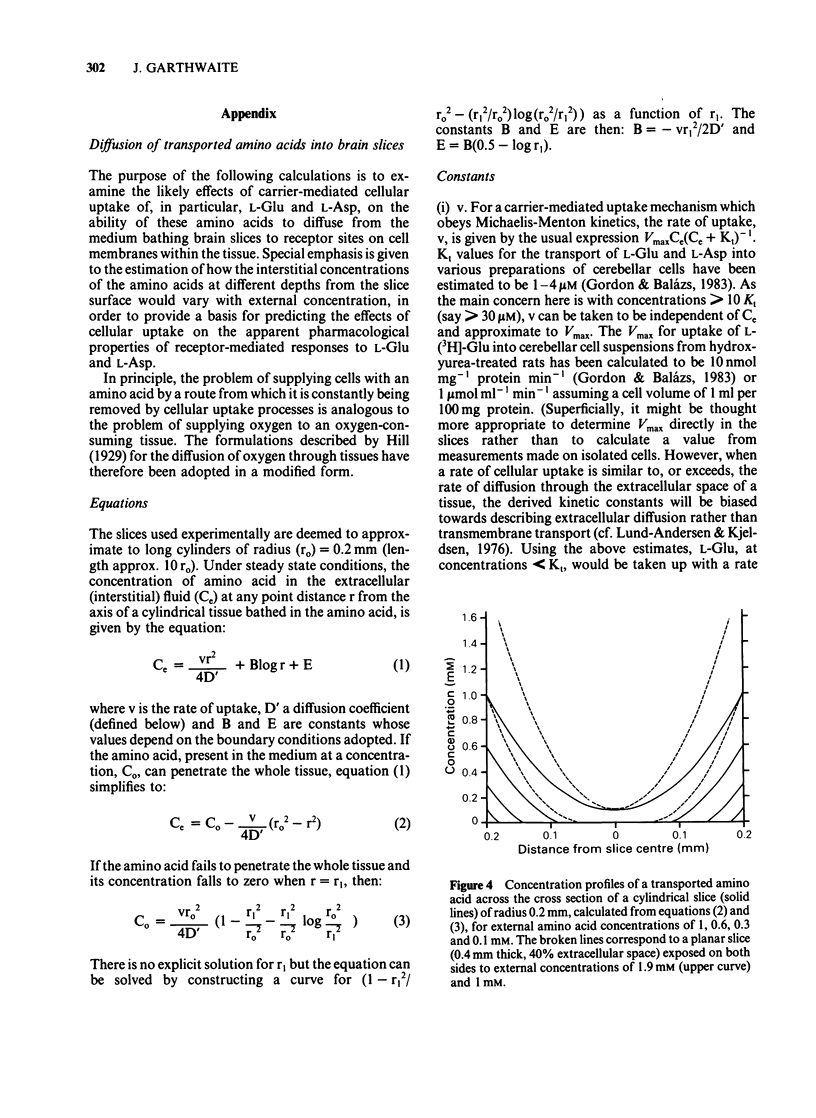
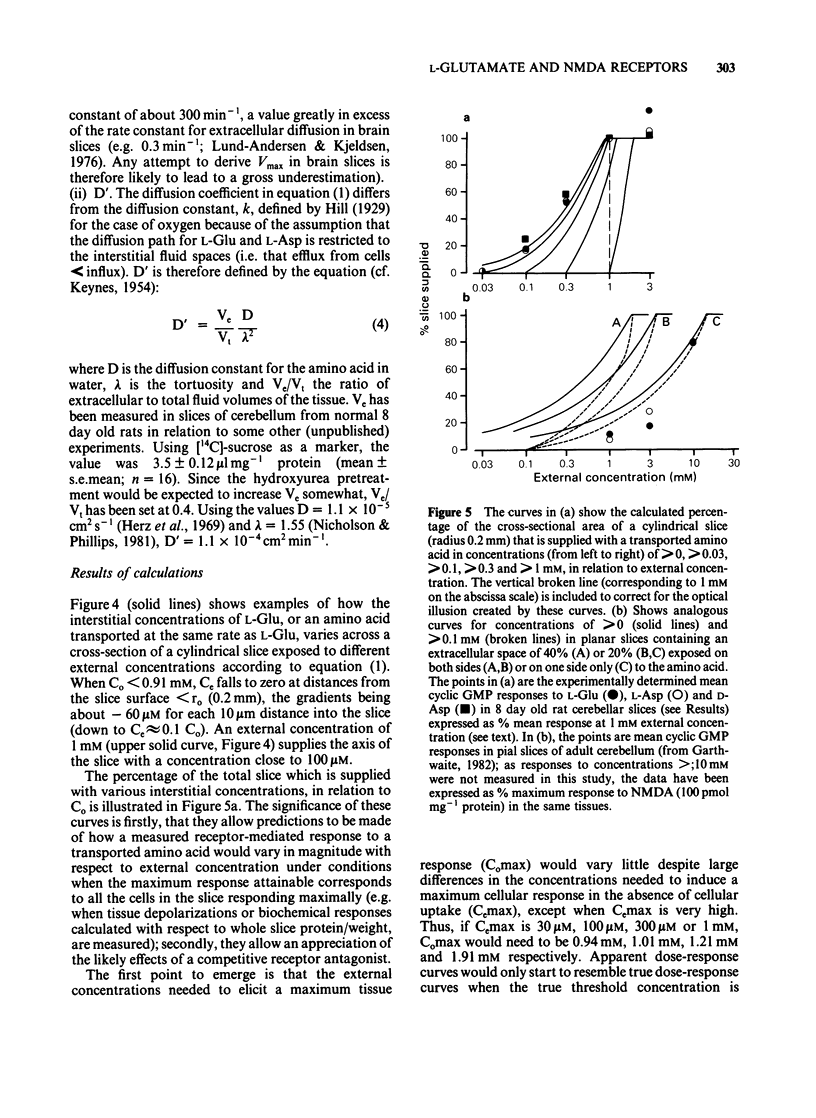
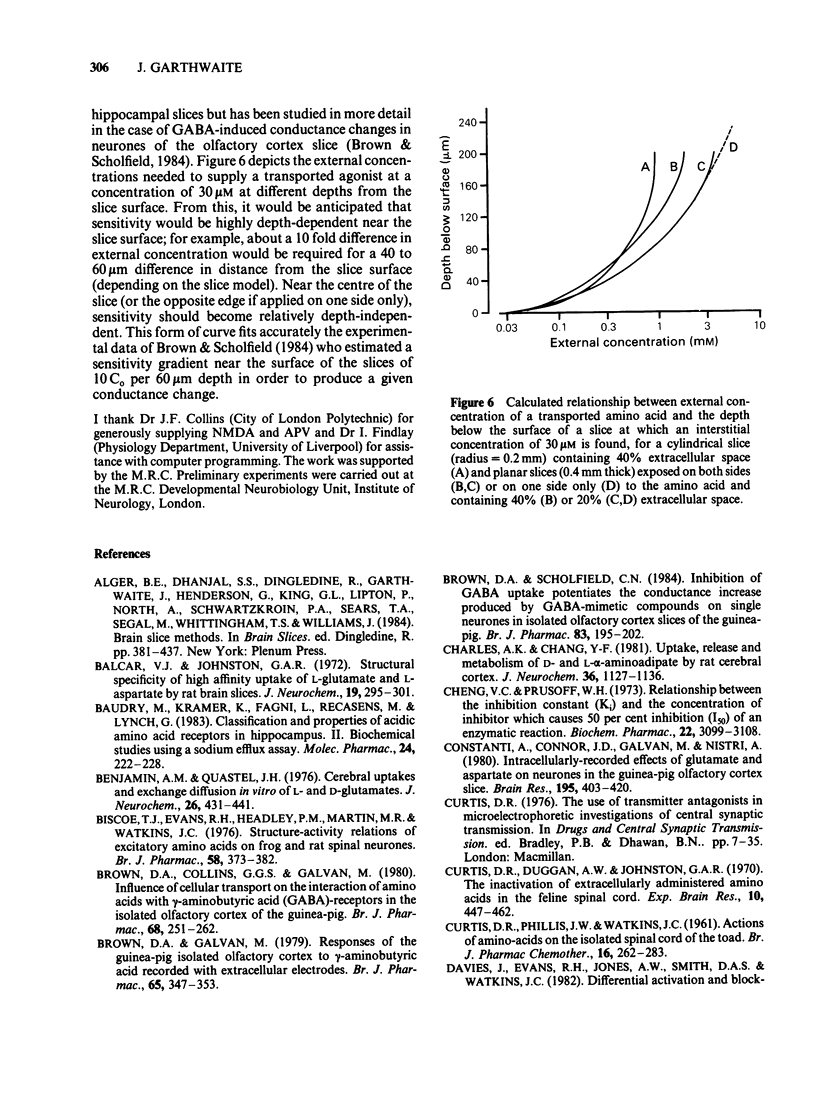
Pharmacological properties of the guanosine 3'5'-cyclic monophosphate (cyclic GMP) responses to excitatory amino acids and their analogues were compared in slices and dissociated cells from the developing rat cerebellum maintained in vitro. The intention was to determine the extent to which cellular uptake might influence the apparent properties of receptor-mediated actions of these compounds. In slices, the potencies of the weakly (or non-) transported analogues, N-methyl-D-aspartate (NMDA) and kainate (KA) (EC50 = 40 microM each) were higher than those of the transported amino acids, D- and L-aspartate (EC50 = 250 microM and 300 microM) and D- and L-glutamate (EC50 = 540 microM and 480 microM). Quisqualate (up to 300 microM) failed to increase cyclic GMP levels significantly. The sensitivity of agonist responses to the NMDA receptor antagonist, DL-2-amino-5-phosphonovalerate (APV), was in the order NMDA greater than L-aspartate greater than L-glutamate, KA. In dissociated cells, L-glutamate was 280 fold more potent (calculated EC50 = 1.7 microM). L- and D-aspartate (calculated EC50 = 13 microM) and D-glutamate (EC50 = 130 microM) were also more effective than in slices. The potencies of NMDA and KA were essentially unchanged. Responses to NMDA, L-glutamate and L-aspartate under these conditions were equally sensitive to inhibition by APV but the response to KA remained relatively resistant to this antagonist. The implications of these results are that, in slices, cellular uptake is responsible for (i) the dose-response curves to L-glutamate, L- and D-aspartate bearing little or no relationship to the true (or relative) potencies of these amino acids; (ii) the potency of APV towards the actions of transported agonists acting at NMDA receptors being reduced and (iii) a differential sensitivity to APV of responses to L-glutamate and L-aspartate being created, the consequence being that a potent action of L-glutamate on NMDA receptors is disguised. These conclusions are supported by theoretical considerations relating to the diffusion of transported amino acids into brain slices, as elaborated in the Appendix.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudry M., Kramer K., Fagni L., Recasens M., Lynch G. Classification and properties of acidic amino acid receptors in hippocampus. II. Biochemical studies using a sodium efflux assay. Mol Pharmacol. 1983 Sep;24(2):222–228. [PubMed] [Google Scholar]
- Benjamin A. M., Quastel J. H. Cerebral uptakes and exchange diffusion in vitro of L- and D-glutamates. J Neurochem. 1976 Mar;26(3):431–441. doi: 10.1111/j.1471-4159.1976.tb01493.x. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Evans R. H., Headley P. M., Martin M. R., Watkins J. C. Structure-activity relations of excitatory amino acids on frog and rat spinal neurones. Br J Pharmacol. 1976 Nov;58(3):373–382. doi: 10.1111/j.1476-5381.1976.tb07714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Collins G. G., Galvan M. Influence of cellular transport on the interaction of amino acids with gamma-aminobutyric acid (GABA)-receptors in the isolated olfactory cortex of the guinea-pig. Br J Pharmacol. 1980 Feb;68(2):251–262. doi: 10.1111/j.1476-5381.1980.tb10414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Galvan M. Responses of the guinea-pig isolated olfactory cortex slice to gamma-aminobutyric acid recorded with extracellular electrodes. Br J Pharmacol. 1979 Feb;65(2):347–353. doi: 10.1111/j.1476-5381.1979.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Inhibition of GABA uptake potentiates the conductance increase produced by GABA-mimetic compounds on single neurones in isolated olfactory cortex slices of the guinea-pig. Br J Pharmacol. 1984 Sep;83(1):195–202. doi: 10.1111/j.1476-5381.1984.tb10135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W., WATKINS J. C. Actions of aminoacids on the isolated hemisected spinal cord of the toad. Br J Pharmacol Chemother. 1961 Jun;16:262–283. doi: 10.1111/j.1476-5381.1961.tb01086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles A. K., Chang Y. F. Uptake, release, and metabolism of D- and L-alpha-aminoadipate by rat cerebral cortex. J Neurochem. 1981 Mar;36(3):1127–1136. doi: 10.1111/j.1471-4159.1981.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Constanti A., Connor J. D., Galvan M., Nistri A. Intracellularly-recorded effects of glutamate and aspartate on neurones in the guinea-pig olfactory cortex slice. Brain Res. 1980 Aug 18;195(2):403–420. doi: 10.1016/0006-8993(80)90075-x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Johnston G. A. The inactivation of extracellularly administered amino acids in the feline spinal cord. Exp Brain Res. 1970 Jun 25;10(5):447–462. doi: 10.1007/BF00234262. [DOI] [PubMed] [Google Scholar]
- Davies J., Evans R. H., Jones A. W., Smith D. A., Watkins J. C. Differential activation and blockade of excitatory amino acid receptors in the mammalian and amphibian central nervous systems. Comp Biochem Physiol C. 1982;72(2):211–224. doi: 10.1016/0306-4492(82)90086-7. [DOI] [PubMed] [Google Scholar]
- Davies L. P., Johnston G. A. Uptake and release of D- and L-aspartate by rat brain slices. J Neurochem. 1976 May;26(5):1007–1014. doi: 10.1111/j.1471-4159.1976.tb06485.x. [DOI] [PubMed] [Google Scholar]
- Del Cerro M. P., Snider R. S., Oster M. Evolution of the extracellular space in immature nervous tissue. Experientia. 1968 Sep 15;24(9):929–930. doi: 10.1007/BF02138661. [DOI] [PubMed] [Google Scholar]
- Dutton G. R., Currie D. N., Tear K. An improved method for the bulk isolation of viable perikarya from postnatal cerebellum. J Neurosci Methods. 1981 Apr;3(4):421–427. doi: 10.1016/0165-0270(81)90029-7. [DOI] [PubMed] [Google Scholar]
- Fagni L., Baudry M., Lynch G. Classification and properties of acidic amino acid receptors in hippocampus. I. Electrophysiological studies of an apparent desensitization and interactions with drugs which block transmission. J Neurosci. 1983 Aug;3(8):1538–1546. doi: 10.1523/JNEUROSCI.03-08-01538.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J., Balázs R. Supersensitivity to the cyclic GMP response to glutamate during cerebellar maturation. Nature. 1978 Sep 28;275(5678):328–329. doi: 10.1038/275328a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Excitatory amino acid receptors and guanosine 3',5'-cyclic monophosphate in incubated slices of immature and adult rat cerebellum. Neuroscience. 1982 Oct;7(10):2491–2497. doi: 10.1016/0306-4522(82)90209-3. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Gilligan G. J. Kainate-glutamate interactions in rat cerebellar slices. Neuroscience. 1984 Jan;11(1):125–138. doi: 10.1016/0306-4522(84)90218-5. [DOI] [PubMed] [Google Scholar]
- Gordon R. D., Balázs R. Characterization of separated cell types from the developing rat cerebellum: transport of glutamate and aspartate by preparations enriched in Purkinje cells, granule neurones, and astrocytes. J Neurochem. 1983 Apr;40(4):1090–1099. doi: 10.1111/j.1471-4159.1983.tb08097.x. [DOI] [PubMed] [Google Scholar]
- Herz A., Zieglgänsberger W., Färber G. Microelectrophoretic studies concerning the spread of glutamic acid and GABA in brain tissue. Exp Brain Res. 1969;9(3):221–235. doi: 10.1007/BF00234456. [DOI] [PubMed] [Google Scholar]
- Ishida A. T., Fain G. L. D-aspartate potentiates the effects of L-glutamate on horizontal cells in goldfish retina. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5890–5894. doi: 10.1073/pnas.78.9.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. A., Kennedy S. M., Twitchin B. Action of the neurotoxin kainic acid on high affinity uptake of L-glutamic acid in rat brain slices. J Neurochem. 1979 Jan;32(1):121–127. doi: 10.1111/j.1471-4159.1979.tb04518.x. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Lodge D., Bornstein J. C., Curtis D. R. Potentiation of L-glutamate and L-aspartate excitation of cat spinal neurones by the stereoisomers of threo-3-hydroxyaspartate. J Neurochem. 1980 Jan;34(1):241–243. doi: 10.1111/j.1471-4159.1980.tb04650.x. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic fluxes in frog muscle. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):359–382. doi: 10.1098/rspb.1954.0030. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lodge D., Headley P. M., Curtis D. R. Selective antagonism by D-alpha-aminoadipate of amino acid and synaptic excitation of cat spinal neurons. Brain Res. 1978 Sep 8;152(3):603–608. doi: 10.1016/0006-8993(78)91117-4. [DOI] [PubMed] [Google Scholar]
- Logan W. J., Snyder S. H. High affinity uptake systems for glycine, glutamic and aspaspartic acids in synaptosomes of rat central nervous tissues. Brain Res. 1972 Jul 20;42(2):413–431. doi: 10.1016/0006-8993(72)90540-9. [DOI] [PubMed] [Google Scholar]
- Lund-Andersen H., Kjeldsen C. S. Uptake of glucose analogues by rat brain cortex slices: a kinetic analysis based upon a model. J Neurochem. 1976 Aug;27(2):361–368. doi: 10.1111/j.1471-4159.1976.tb12254.x. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Mixed-agonist action of excitatory amino acids on mouse spinal cord neurones under voltage clamp. J Physiol. 1984 Sep;354:29–53. doi: 10.1113/jphysiol.1984.sp015360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan H. Receptors for the excitatory amino acids in the mammalian central nervous system. Prog Neurobiol. 1983;20(3-4):251–271. doi: 10.1016/0301-0082(83)90004-7. [DOI] [PubMed] [Google Scholar]
- Nicholson C., Phillips J. M. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J Physiol. 1981 Dec;321:225–257. doi: 10.1113/jphysiol.1981.sp013981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Quastel J. H. Spontaneous action potentials in isolated guinea-pig cerebellar slices: effects of amino acids and conditions affecting sodium and water uptake. Proc R Soc Lond B Biol Sci. 1973 Aug 31;184(1074):83–90. doi: 10.1098/rspb.1973.0032. [DOI] [PubMed] [Google Scholar]
- Olverman H. J., Jones A. W., Watkins J. C. L-glutamate has higher affinity than other amino acids for [3H]-D-AP5 binding sites in rat brain membranes. Nature. 1984 Feb 2;307(5950):460–462. doi: 10.1038/307460a0. [DOI] [PubMed] [Google Scholar]
- Peet M. J., Leah J. D., Curtis D. R. Antagonists of synaptic and amino acid excitation of neurones in the cat spinal cord. Brain Res. 1983 Apr 25;266(1):83–95. doi: 10.1016/0006-8993(83)91311-2. [DOI] [PubMed] [Google Scholar]
- Skerritt J. H., Johnston G. A. Uptake and release of N-methyl-D-aspartate by rat brain slices. J Neurochem. 1981 Mar;36(3):881–885. doi: 10.1111/j.1471-4159.1981.tb01676.x. [DOI] [PubMed] [Google Scholar]
- Teichberg V. I., Goldberg O., Luini A. The stimulation of ion fluxes in brain slices by glutamate and other excitatory amino acids. Mol Cell Biochem. 1981 Sep 25;39:281–295. doi: 10.1007/BF00232580. [DOI] [PubMed] [Google Scholar]
- VERNADAKIS A., WOODBURY D. M. CELLULAR AND EXTRACELLULAR SPACES IN DEVELOPING RAT BRAIN. RADIOACTIVE UPTAKE STUDIES WITH CHLORIDE AND INULIN. Arch Neurol. 1965 Mar;12:284–293. doi: 10.1001/archneur.1965.00460270060008. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Watkins J. C. Pharmacology of excitatory amino acid transmitters. Adv Biochem Psychopharmacol. 1981;29:205–212. [PubMed] [Google Scholar]
- Wilkin G. P., Balázs R., Wilson J. E., Cohen J., Dutton G. R. Preparation of cell bodies from the developing cerebellum: structural and metabolic integrity of the isolated cells. Brain Res. 1976 Oct 15;115(2):181–199. doi: 10.1016/0006-8993(76)90506-0. [DOI] [PubMed] [Google Scholar]


