Abstract
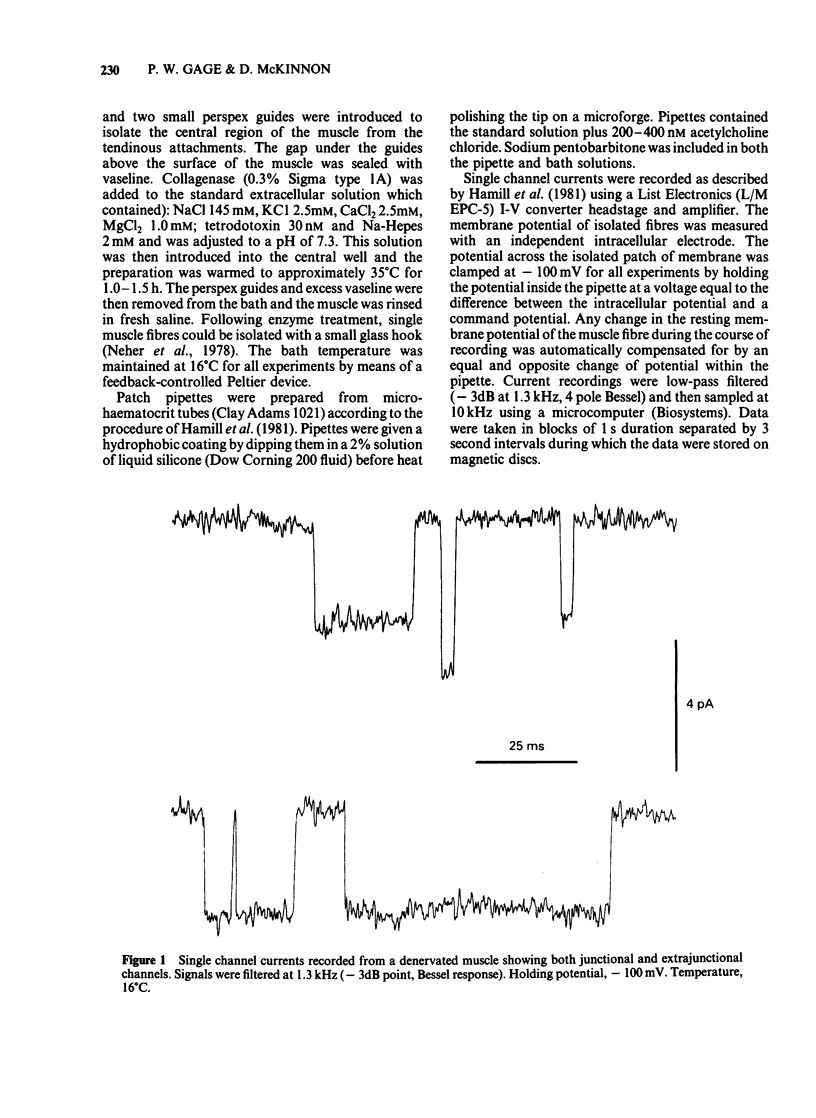
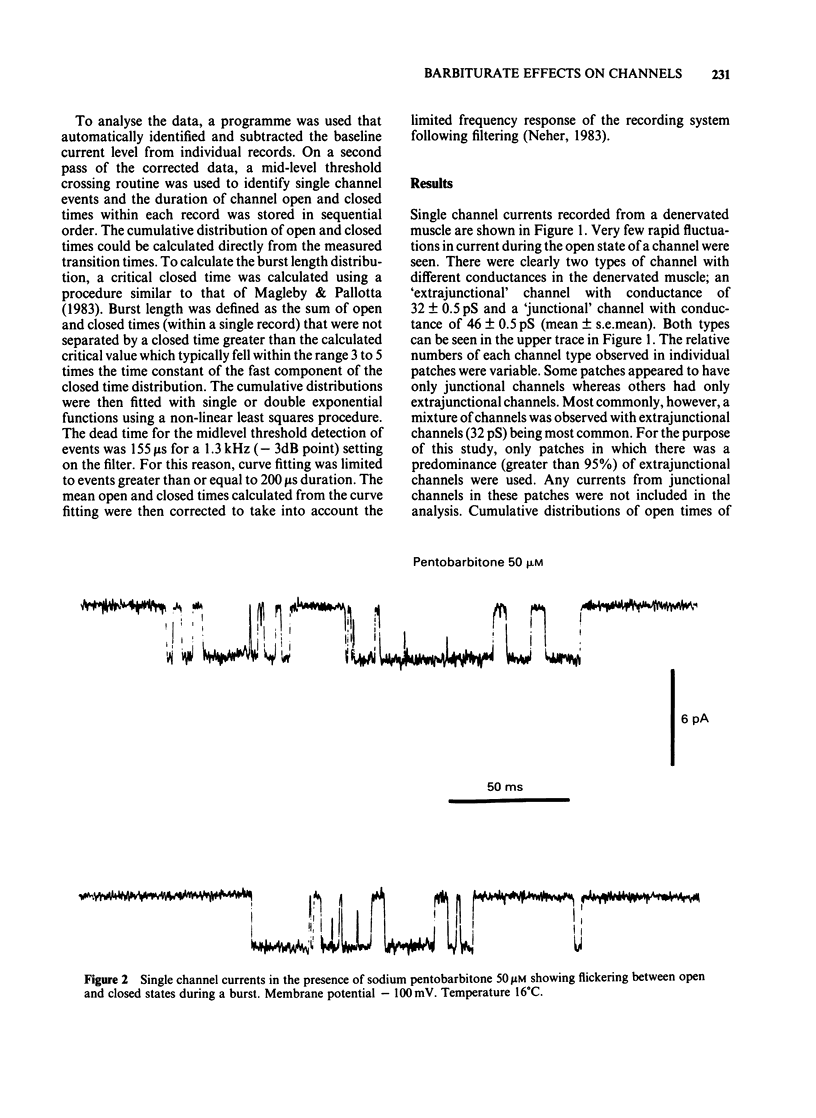
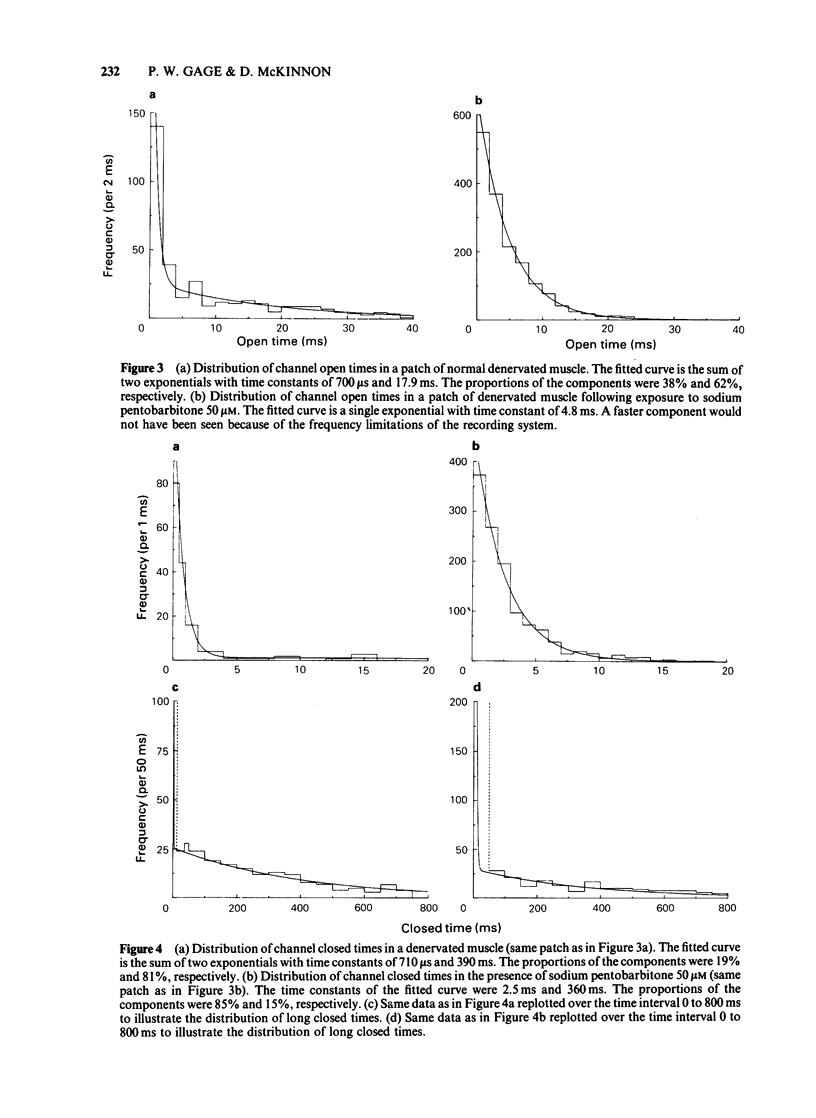
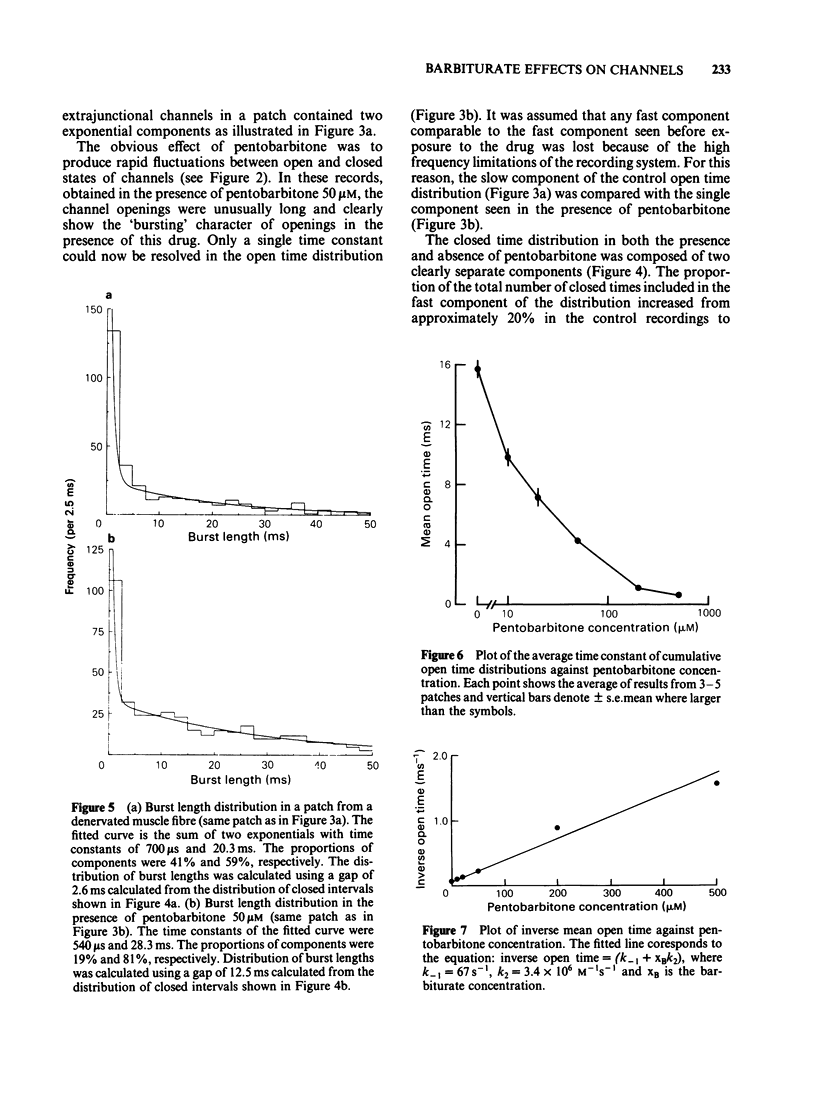
Acetylcholine-activated single channel currents were recorded from the extrajunctional region of chronically denervated skeletal muscle of the rat by the patch clamp technique. In control experiments, the cumulative open-time, closed-time and burst length distributions could be well described by the sum of two exponentials. Pentobarbitone decreased the mean open time and increased the time constant of the fast component of the closed time distribution. These effects increased with drug concentration. The mean burst length was relatively independent of pentobarbitone concentration over the range of concentrations used (10-500 microM). These observations are inconsistent with a simple sequential blocking model and it is suggested that pentobarbitone has an allosteric site of action on receptor-channel complexes that makes the open state less stable.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Proceedings: The mechanism by which amylobarbitone and thiopentone block the end-plate response to nicotinic agonists. J Physiol. 1974 Aug;241(1):41P–42P. [PubMed] [Google Scholar]
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Do general anaesthetics act by competitive binding to specific receptors? Nature. 1984 Aug 16;310(5978):599–601. doi: 10.1038/310599a0. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Hamill O. P., Wachtel R. E. Sites of action of procaine at the motor end-plate. J Physiol. 1983 Feb;335:123–137. doi: 10.1113/jphysiol.1983.sp014524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Sah P. Postsynaptic effects of some central stimulants at the neuromuscular junction. Br J Pharmacol. 1982 Mar;75(3):493–502. doi: 10.1111/j.1476-5381.1982.tb09166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Wachtel R. E. Some effects of procaine at the toad end-plate are not consistent with a simple channel-blocking model. J Physiol. 1984 Jan;346:331–339. doi: 10.1113/jphysiol.1984.sp015025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. Burst kinetics of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1983 Nov;344:605–623. doi: 10.1113/jphysiol.1983.sp014958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B., Steinbach J. H. The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 1978 Jul 18;375(2):219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyama I., Narahashi T. Mechanism of blockade of neuromuscular transmission by pentobarbital. J Pharmacol Exp Ther. 1975 Jan;192(1):95–104. [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of a nicotinic acetylcholine receptor. Biophys J. 1984 Jan;45(1):175–185. doi: 10.1016/S0006-3495(84)84146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach A. B. A kinetic model for the action of xylocaine on receptors for acetylcholine. J Gen Physiol. 1968 Jul;52(1):162–180. doi: 10.1085/jgp.52.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torda T. A., Gage P. W. Effect of barbiturates on synaptic currents. Anaesth Intensive Care. 1976 Aug;4(3):199–202. doi: 10.1177/0310057X7600400305. [DOI] [PubMed] [Google Scholar]


