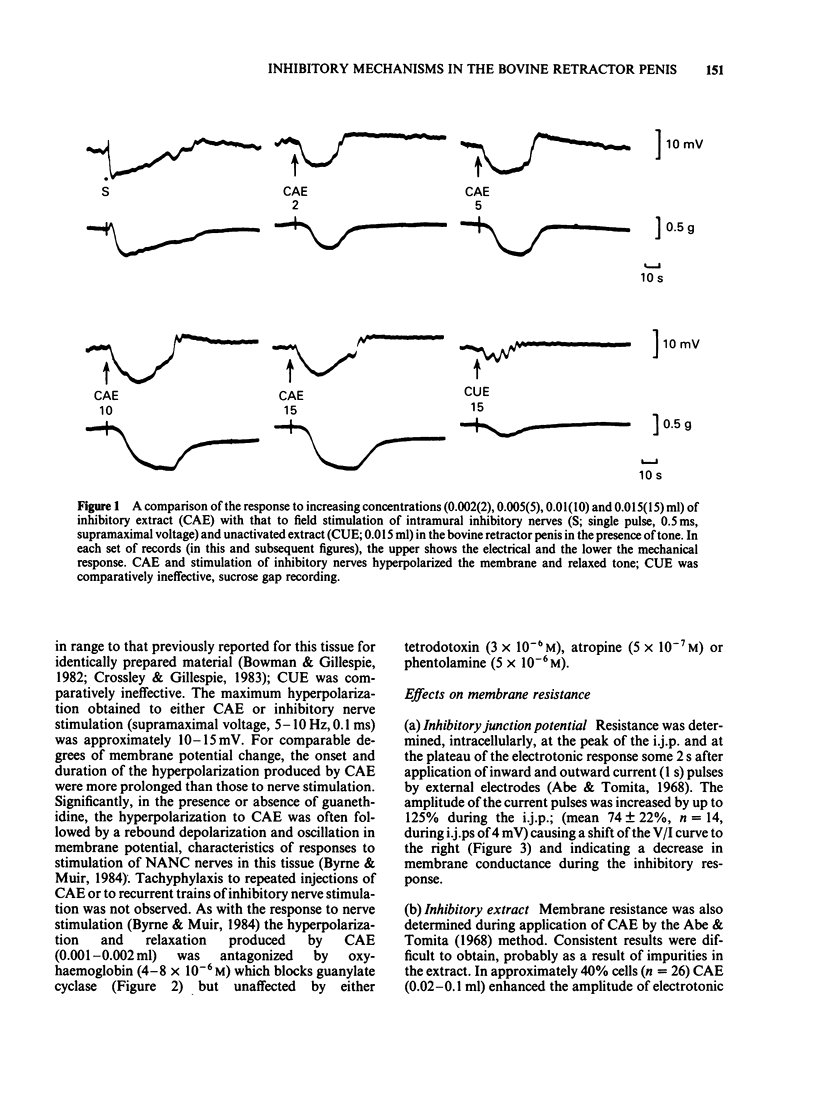
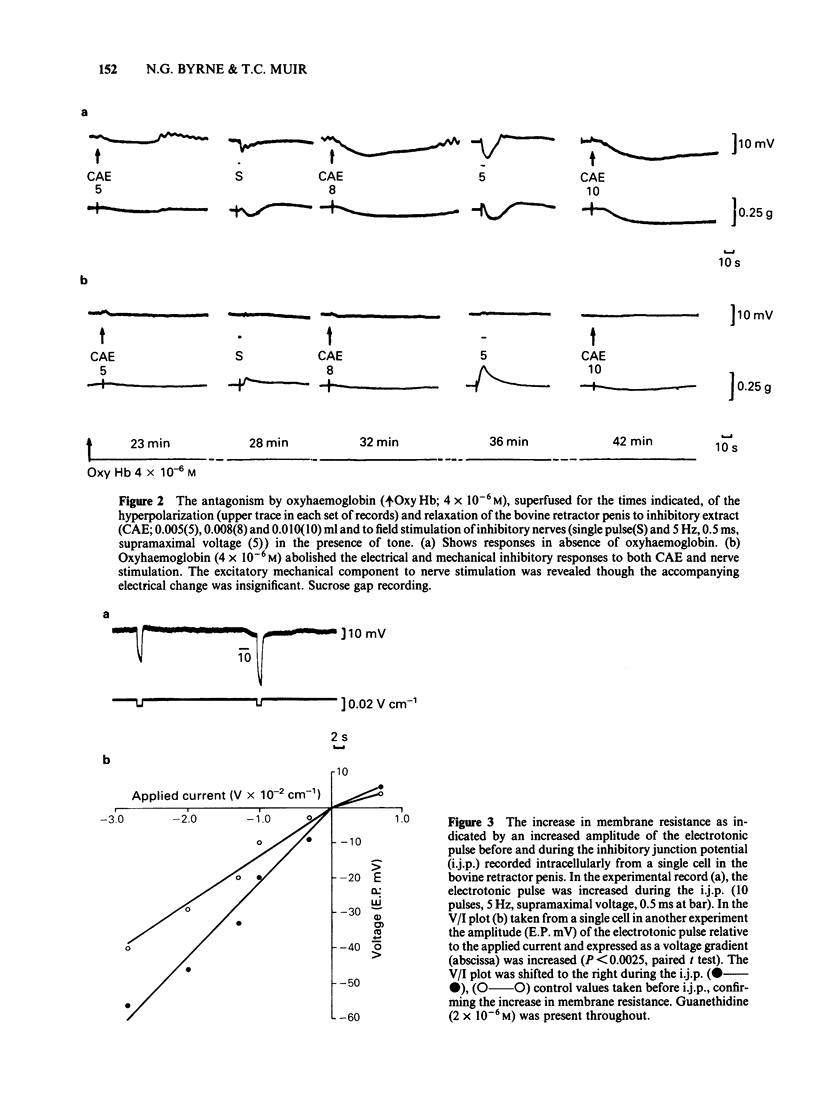
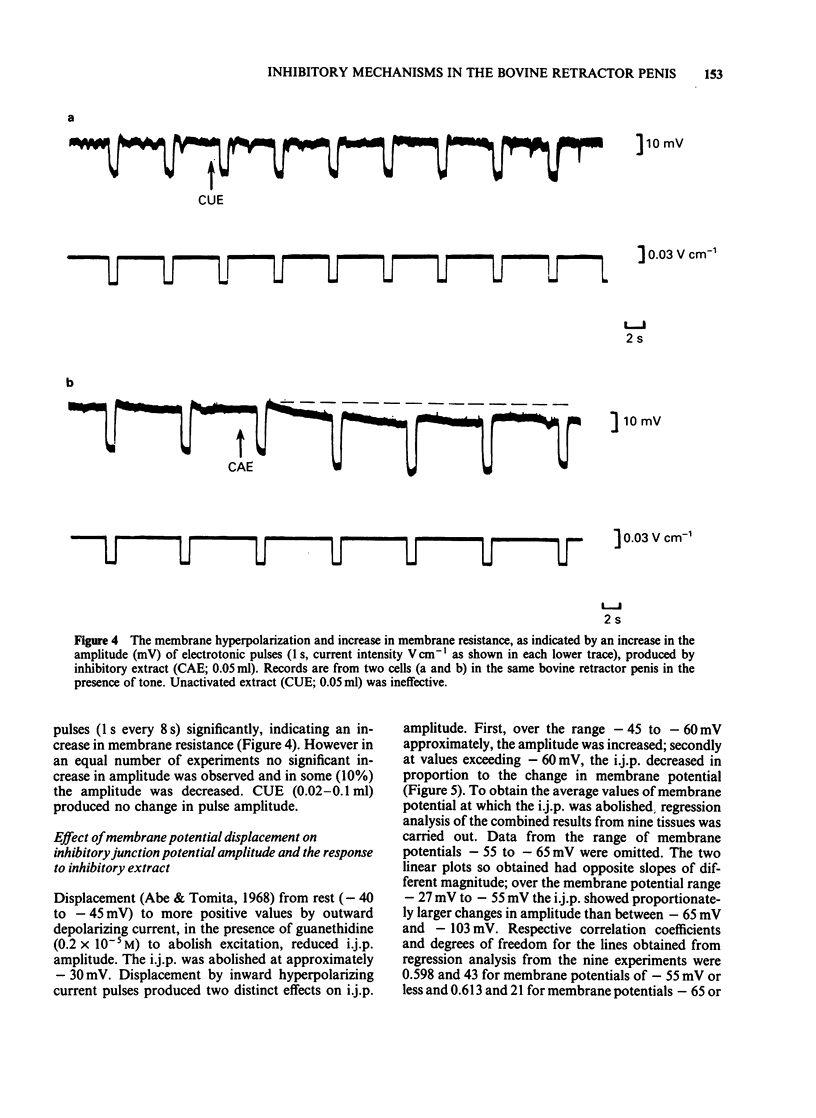
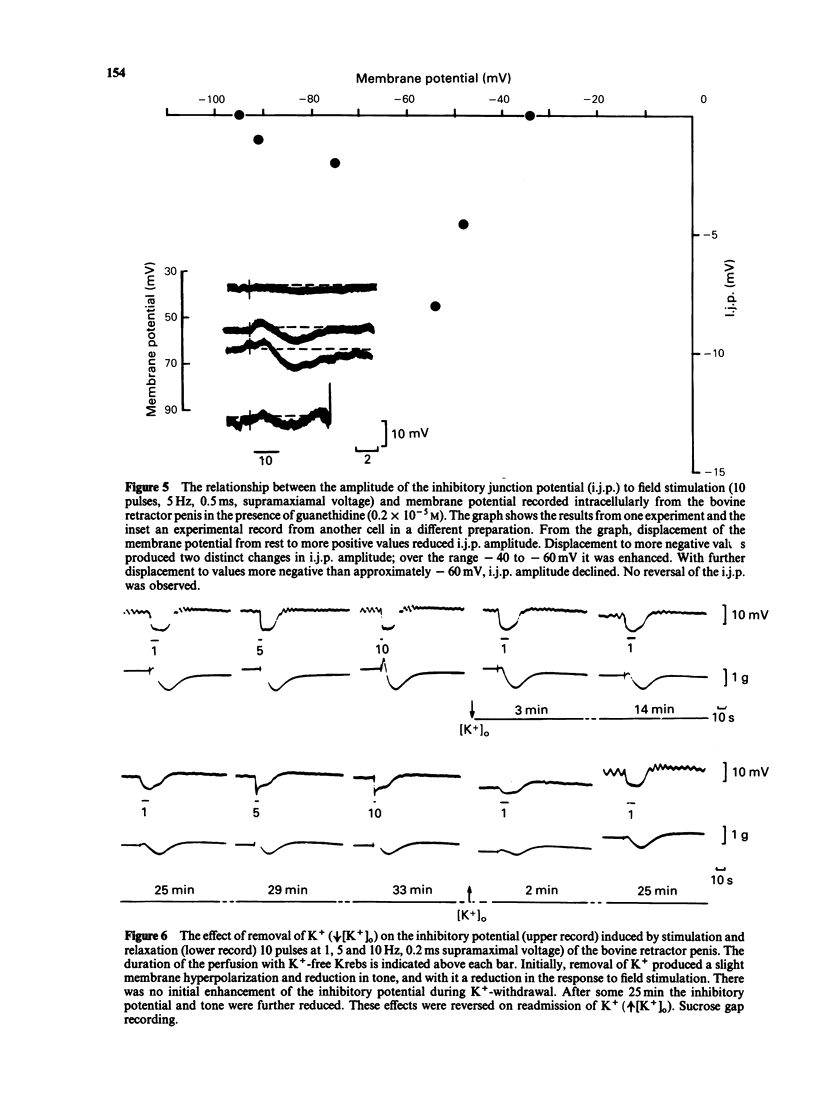
Abstract
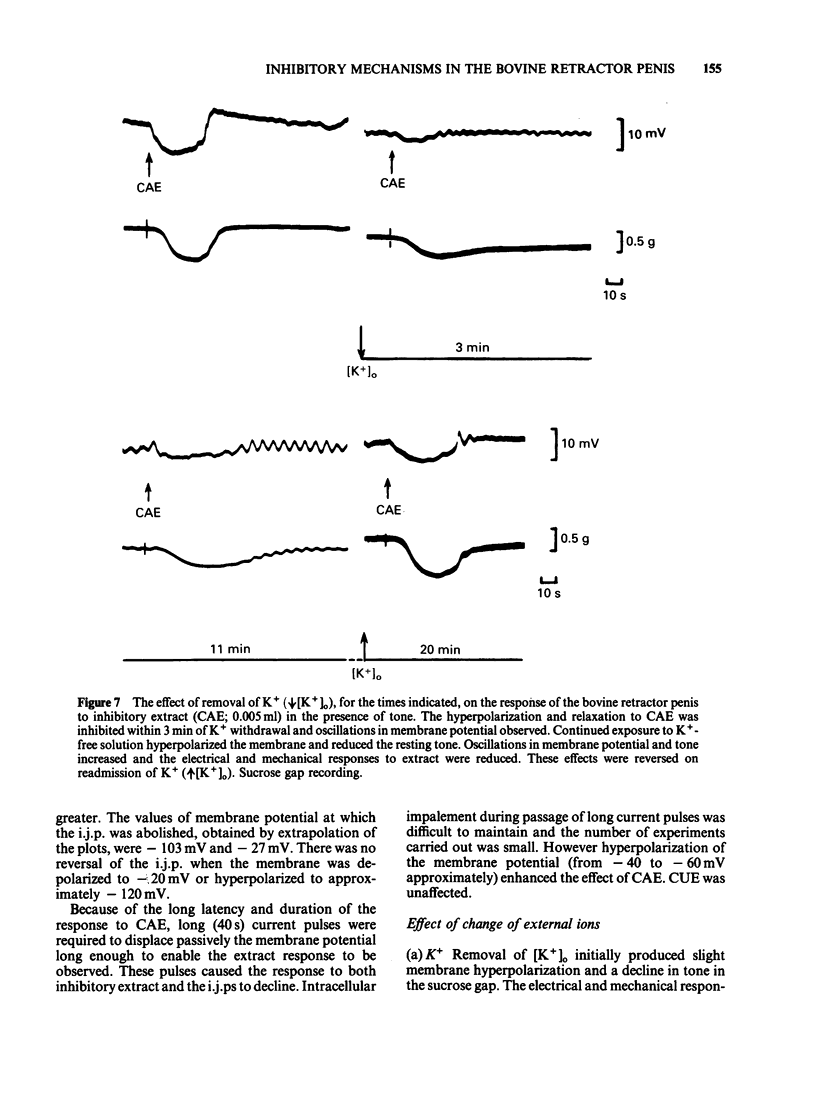
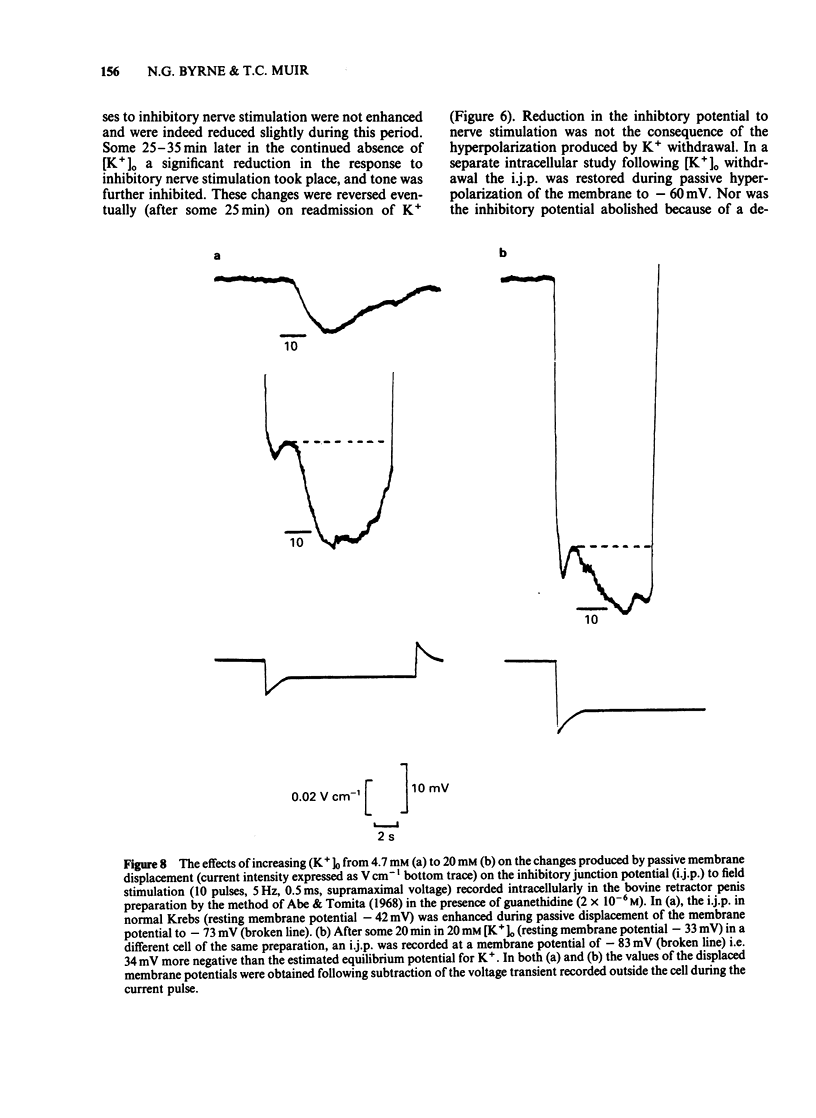
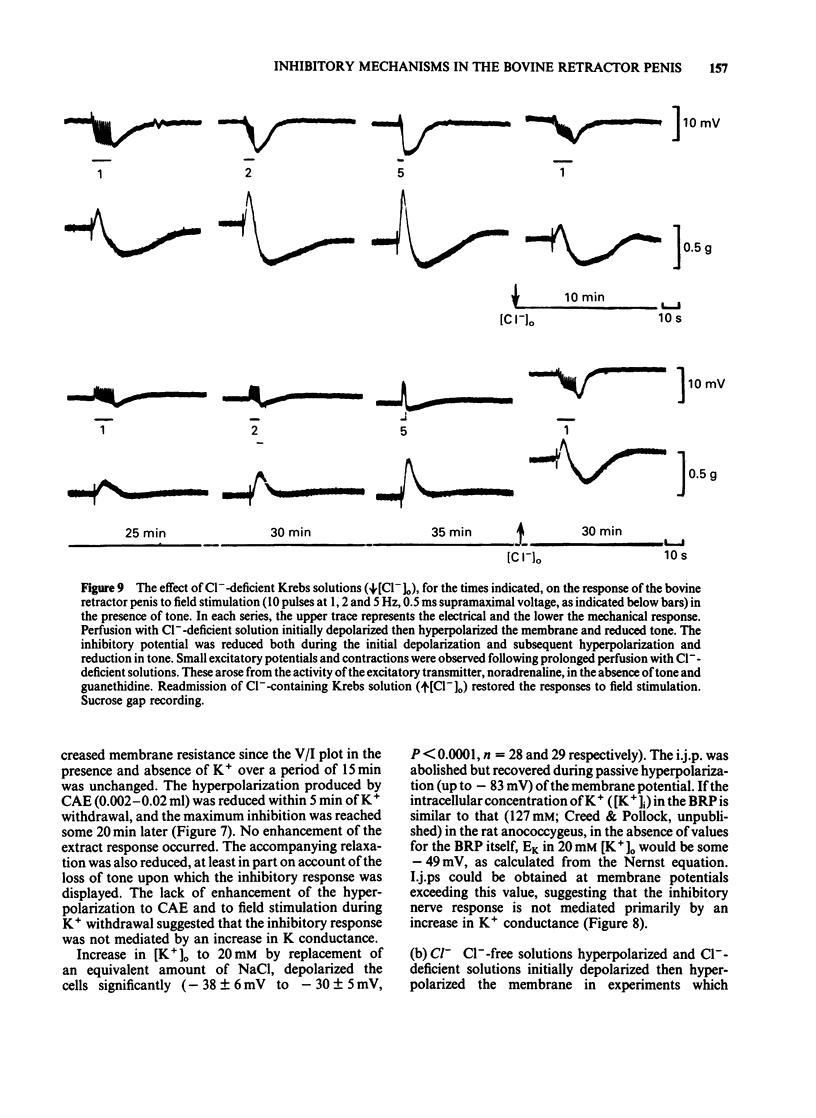
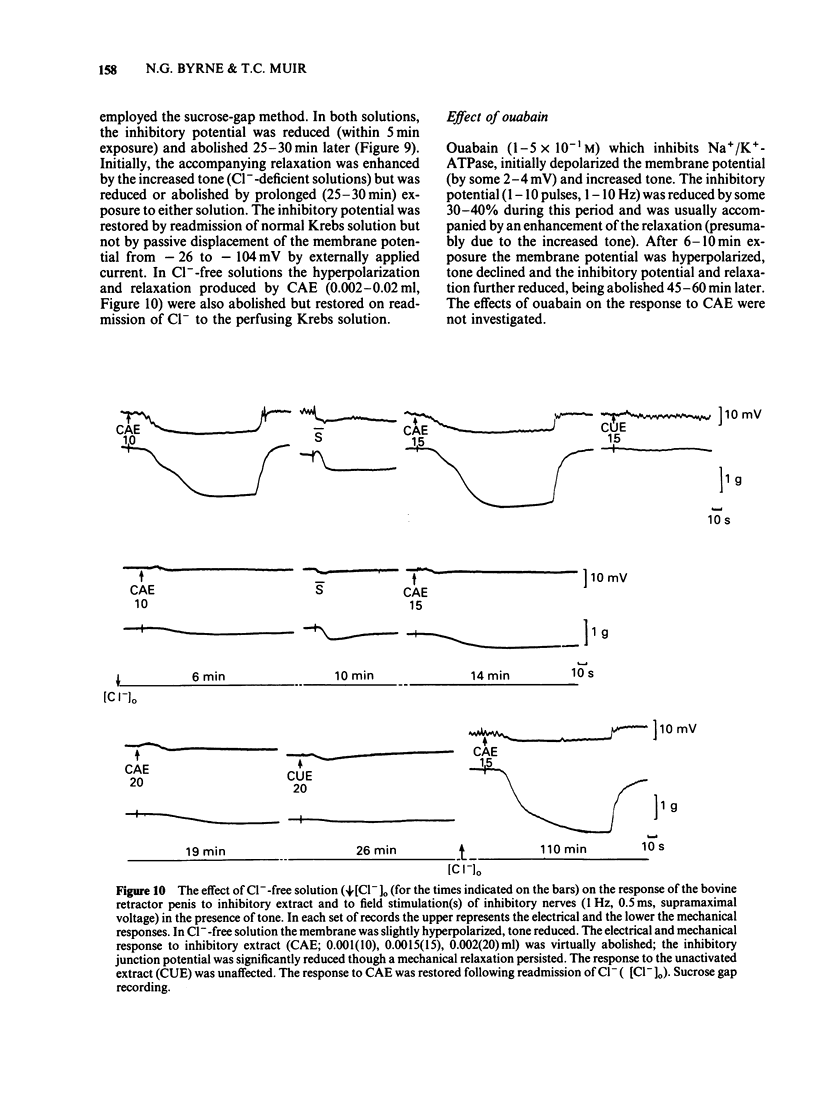
The response of the bovine retractor penis (BRP) to stimulation of non-adrenergic, non-cholinergic (NANC) inhibitory nerves and to an inhibitory extract prepared from this muscle have been studied using intracellular microelectrode, sucrose gap and conventional mechanical recording techniques. Both inhibitory nerve stimulation and inhibitory extract hyperpolarized the membrane potential and relaxed spontaneous or guanethidine (3 X 10(-5) M)-induced tone. These effects were accompanied by an increase in membrane resistance. Following membrane potential displacement from an average value of -53 +/- 7 mV (n = 184; Byrne & Muir, 1984) inhibitory potentials to nerve stimulation were abolished at approximately -30 mV; there was no evidence of reversal. Displacement by inward hyperpolarizing current over the range -45 to -60 mV increased the inhibitory response to nerve stimulation and to inhibitory extract; at more negative potential values (above approximately -60 mV) the inhibitory potential decreased and was abolished (approximately -103 mV). There was no evidence of reversal. Removal of [K+]o reversibly reduced hyperpolarization to nerve stimulation and inhibitory extract. No enhancement was observed. Increasing the [K+]o to 20 mM reduced the inhibitory potential to nerve stimulation but this was restored by passive membrane hyperpolarization. Inhibitory potentials were obtained at membrane potential values exceeding that of the estimated EK (-49 mV). [Cl-]o-free or [Cl-]o-deficient solutions reduced and abolished (after some 20-25 min) the hyperpolarization produced by inhibitory nerve stimulation or inhibitory extract. The inhibitory potential amplitude following nerve stimulation was not restored by passive displacement of the membrane potential from -26 to -104 mV approximately. Ouabain (1-5 X 10(-5) M) reduced then (45-60 min later) abolished the inhibitory potential to nerve stimulation. The effects of this drug on the extract were not investigated. It is concluded that the inhibitory response to nerve stimulation and extract in the BRP may involve several ionic species. However, unlike that in gastrointestinal muscles the NANC response in the BRP is accompanied by an increased membrane resistance and does not primarily involve K+. The underlying mechanisms for the inhibitory response to both NANC nerve stimulation and inhibitory extract appear to be similar, compatible with the view that the latter may contain the inhibitory transmitter released from these nerves in this tissue.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. Measurement of intracellular chloride in guinea-pig vas deferens by ion analysis, 36chloride efflux and micro-electrodes. J Physiol. 1982 May;326:139–154. doi: 10.1113/jphysiol.1982.sp014182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C., Brading A. F. Towards an estimate of chloride permeability in the smooth muscle of guinea-pig vas deferens. J Physiol. 1983 Mar;336:179–197. doi: 10.1113/jphysiol.1983.sp014575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer V., Kuriyama H. The nature of non-cholinergic, non-adrenergic transmission in longitudinal and circular muscles of the guinea-pig ileum. J Physiol. 1982 Nov;332:375–391. doi: 10.1113/jphysiol.1982.sp014419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Drummond A. H. Cyclic GMP mediates neurogenic relaxation in the bovine retractor penis muscle. Br J Pharmacol. 1984 Apr;81(4):665–674. doi: 10.1111/j.1476-5381.1984.tb16133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S. Block of some non-adrenergic inhibitory responses of smooth muscle by a substance from haemolysed erythrocytes. J Physiol. 1982 Jul;328:11–25. doi: 10.1113/jphysiol.1982.sp014250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Martin W. The inhibitory material in extracts from the bovine retractor penis muscle is not an adenine nucleotide. Br J Pharmacol. 1979 Nov;67(3):327–328. doi: 10.1111/j.1476-5381.1979.tb08683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne N. G., Muir T. C. Electrical and mechanical responses of the bovine retractor penis to nerve stimulation and to drugs. J Auton Pharmacol. 1984 Dec;4(4):261–271. doi: 10.1111/j.1474-8673.1984.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Bywater R. A., Taylor G. S. The passive membrane properties and excitatory junction potentials of the guinea pig deferens. J Physiol. 1980 Mar;300:303–316. doi: 10.1113/jphysiol.1980.sp013163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E., Gillespie J. S., Muir T. C. The electrical basis of excitation and inhibition in the rat anoccygeus muscle. J Physiol. 1975 Feb;245(1):33–47. doi: 10.1113/jphysiol.1975.sp010833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley A. W., Gillespie J. S. The effect of an inhibitory factor from the bovine retractor penis on the gastro-intestinal tract and gall bladder of the guinea-pig. Br J Pharmacol. 1983 Jan;78(1):213–220. doi: 10.1111/j.1476-5381.1983.tb09382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Excitation of the crayfish neuromuscular junction with decreased membrane conductance. Nature. 1960 Jul 16;187:246–247. doi: 10.1038/187246a0. [DOI] [PubMed] [Google Scholar]
- Engberg I., Marshall K. C. Mechanism of noradrenaline hyperpolarization in spinal cord motoneurones of the cat. Acta Physiol Scand. 1971 Sep;83(1):142–144. doi: 10.1111/j.1748-1716.1971.tb05061.x. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Hunter J. C., Martin W. Some physical and chemical properties of the smooth muscle inhibitory factor in extracts of the bovine retractor penis muscle. J Physiol. 1981 Jun;315:111–125. doi: 10.1113/jphysiol.1981.sp013736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. The chloride conductance of frog skeletal muscle. J Physiol. 1960 Apr;151:89–102. [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka T., Kuriyama H. Responses of the smooth muscle membrane of guinea pig jejunum elicited by field stimulation. J Gen Physiol. 1969 Apr;53(4):471–486. doi: 10.1085/jgp.53.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager L. P., Schevers J. A. A comparison of effects evoked in guinea-pig taenia caecum by purine nucleotides and by "purinergic" nerve stimulation. J Physiol. 1980 Feb;299:75–83. doi: 10.1113/jphysiol.1980.sp013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas A. J. The effects of apamin on responses evoked by field stimulation in guinea-pig taenia caeci. Eur J Pharmacol. 1981 Jul 17;73(1):1–9. doi: 10.1016/0014-2999(81)90139-4. [DOI] [PubMed] [Google Scholar]
- Shuba M. F. The mechanism of the excitatory action of catecholamines and histamine on the smooth muscle of guinea-pig ureter. J Physiol. 1977 Jan;264(3):853–864. doi: 10.1113/jphysiol.1977.sp011698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Conductance change during the inhibitory potential in the guinea-pig taenia coli. J Physiol. 1972 Sep;225(3):693–703. doi: 10.1113/jphysiol.1972.sp009964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weight F. F., Padjen A. Slow synaptic inhibition: evidence for synaptic inactivation of sodium conductance in sympathetic ganglion cells. Brain Res. 1973 May 30;55(1):219–224. doi: 10.1016/0006-8993(73)90505-2. [DOI] [PubMed] [Google Scholar]
- den Hertog A., Jager L. P. Ion fluxes during the inhibitory junction potential in the guinea-pig taenia coli. J Physiol. 1975 Sep;250(3):681–691. doi: 10.1113/jphysiol.1975.sp011077. [DOI] [PMC free article] [PubMed] [Google Scholar]


