Abstract
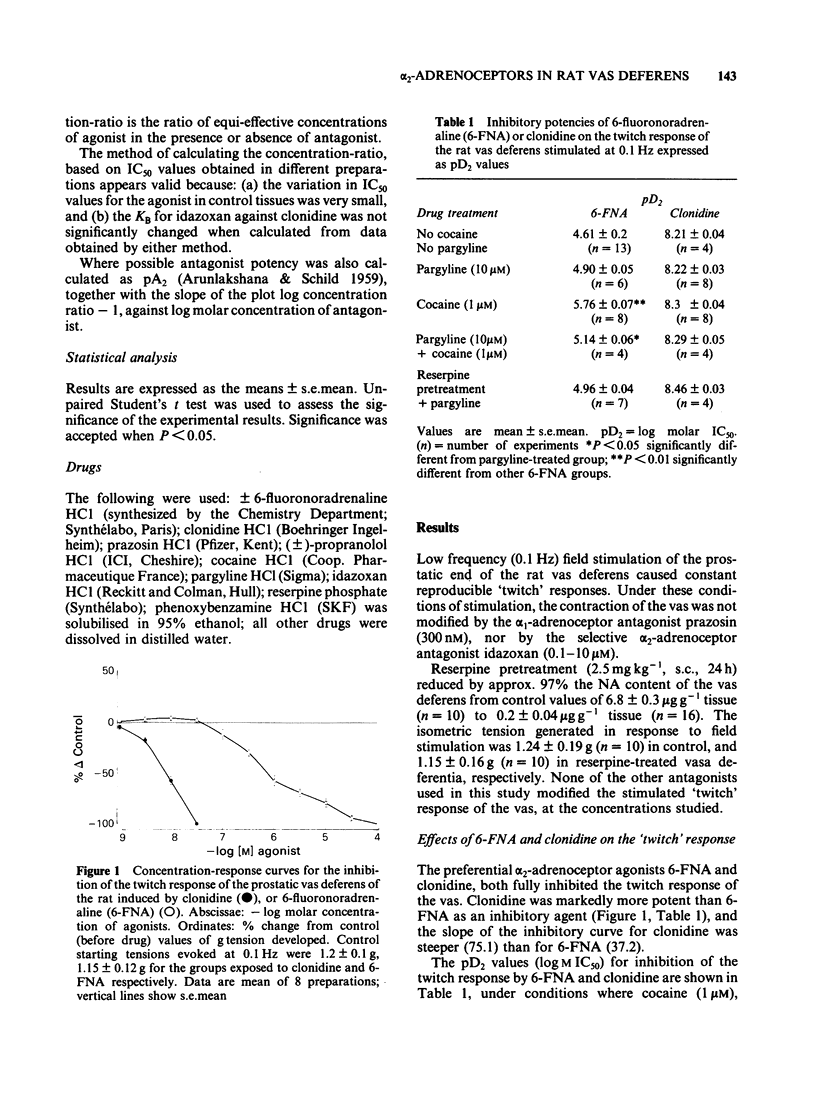
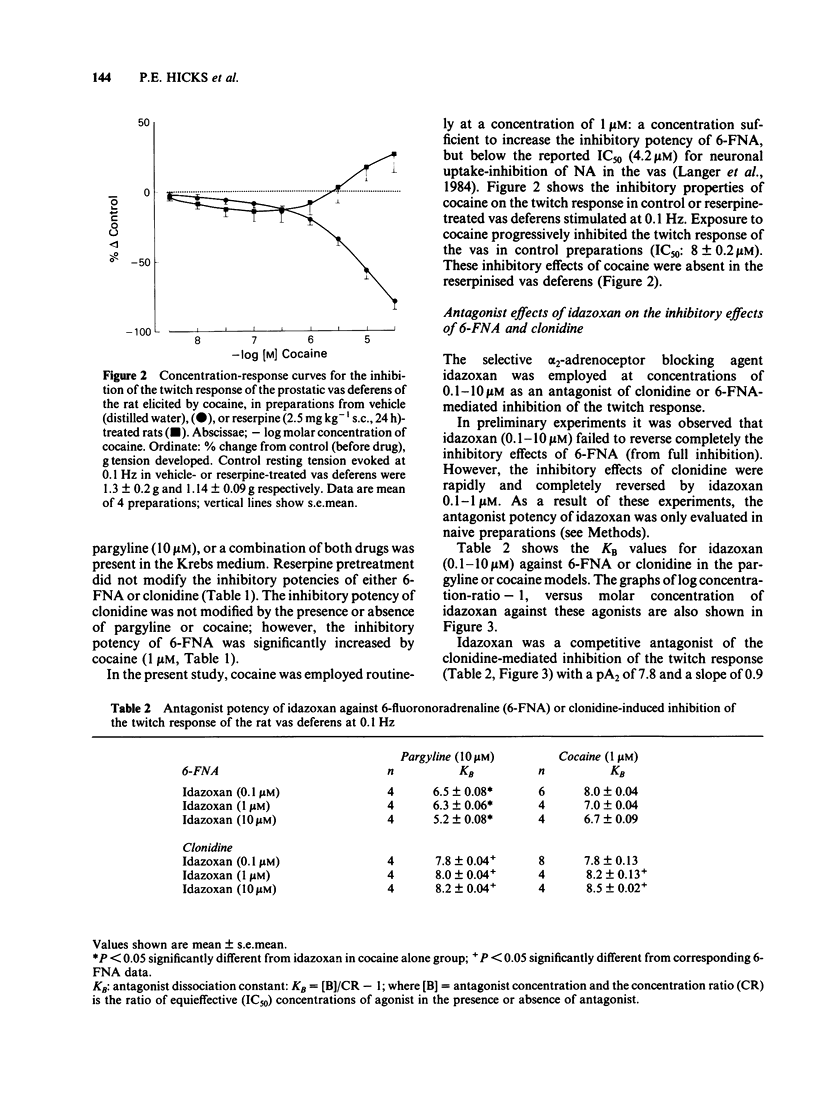
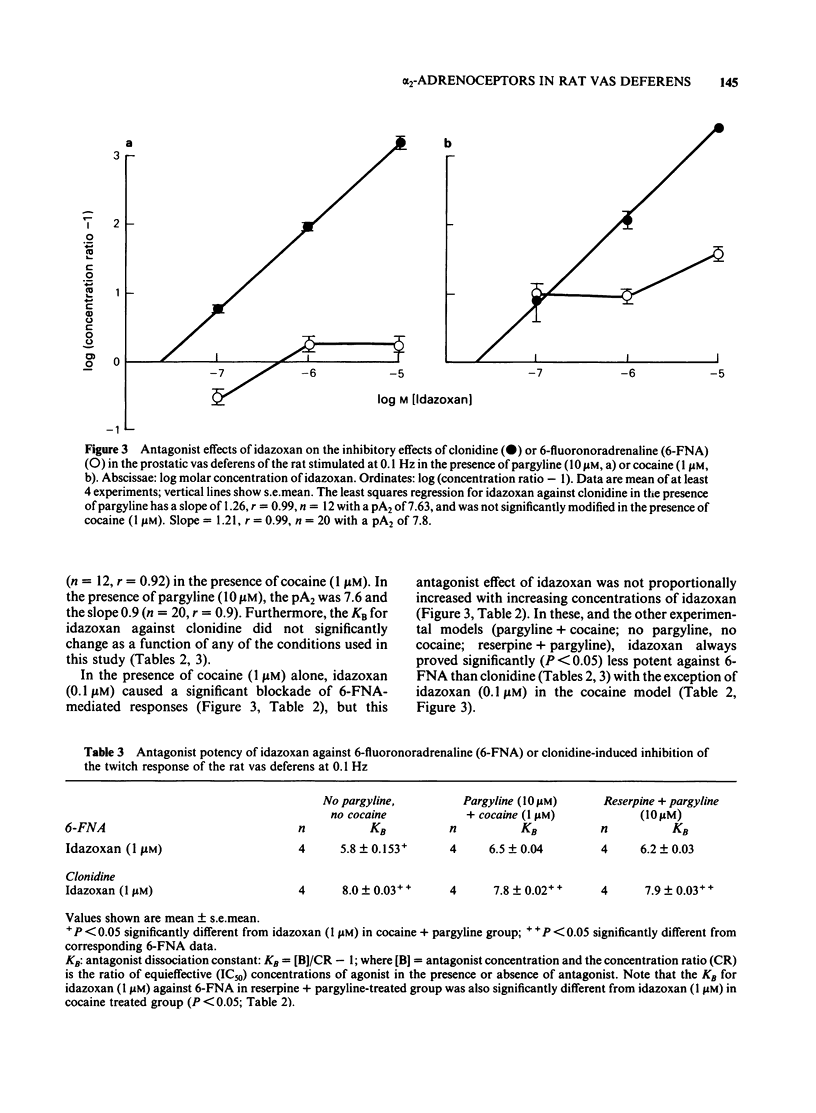
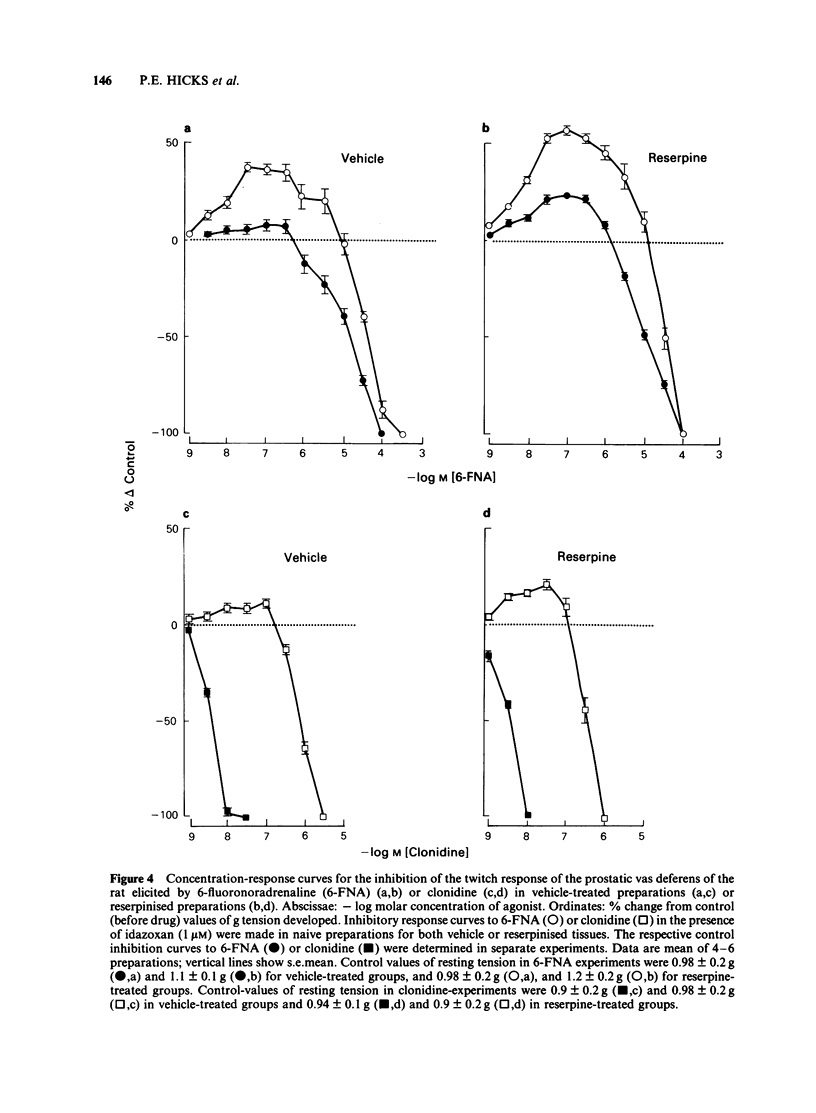
The prejunctional inhibitory effects of clonidine and 6-fluoronoradrenaline (6-FNA) have been evaluated in the isolated prostatic segment of the rat vas deferens, against the twitch response evoked by low frequency (0.1 Hz) field stimulation. The inhibitory potency of 6FNA was significantly increased in the presence of cocaine (1 microM) or pargyline (10 microM), but was not modified in the vas deferens from rats pretreated with reserpine when the endogenous levels of noradrenaline (NA) were decreased by 97%. Clonidine was significantly more potent than 6-FNA as an inhibitory agonist, and the potency of clonidine was not modified after cocaine, pargyline or reserpine. The alpha 2-adrenoceptor blocking agent idazoxan, was a competitive antagonist against the inhibitory effects of clonidine under all experimental conditions. In contrast, the only antagonism shown by idazoxan against the inhibitory effects of 6-FNA was in the presence of cocaine (1 microM), and this antagonist effect of idazoxan was not concentration-related. Low concentrations of 6-FNA caused concentration-dependent facilitatory effects on the twitch response, which were significantly greater after treatment with idazoxan (1 microM) in reserpine-treated vas deferens. These facilitatory effects of 6-FNA were always observed in the presence of prazosin (300 nM) and also after treatment of the preparations with phenoxybenzamine (10 microM), a concentration which abolished the inhibitory actions of both clonidine and 6-FNA. The facilitatory effects on the twitch response induced by low concentrations of 6-FNA are therefore unlikely to be due to either alpha 1- or alpha 2-adrenoceptor stimulation. In conclusion, the failure of idazoxan to block the inhibitory effects of 6-FNA, while exerting a potent competitive antagonism of clonidine-induced inhibitory effects, supports the proposal that alpha 2-adrenoceptors may in fact be subdivided into two subclasses, involving imidazoline and phenylethylamine recognition sites.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambache N., Zar M. A. Evidence against adrenergic motor transmission in the guinea-pig vas deferens. J Physiol. 1971 Jul;216(2):359–389. doi: 10.1113/jphysiol.1971.sp009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton P. G., Duncan M. E., McGrath J. C. An analysis of the anatomical basis for the mechanical response to motor nerve stimulation of the rat vas deferens. J Physiol. 1977 Dec;273(1):23–43. doi: 10.1113/jphysiol.1977.sp012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. J., Drew G. M., Hilditch A. Presynaptic alpha-adrenoceptors: do exogenous and neuronally released noradrenaline act at different sites? Br J Pharmacol. 1984 Mar;81(3):457–464. doi: 10.1111/j.1476-5381.1984.tb10098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet P., Feldman J., Schwartz J. Central cardiovascular effects of alpha adrenergic drugs: differences between catecholamines and imidazolines. J Pharmacol Exp Ther. 1984 Jul;230(1):232–236. [PubMed] [Google Scholar]
- Brown C. M., McGrath J. C., Summers R. J. The effects of alpha-adrenoceptor agonists and antagonists on responses of transmurally stimulated prostatic and epididymal portions of the isolated vas deferens of the rat. Br J Pharmacol. 1979 Aug;66(4):553–564. doi: 10.1111/j.1476-5381.1979.tb13694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroon J. M., Clark R. D., Kluge A. F., Olah R., Repke D. B., Unger S. H., Michel A. D., Whiting R. L. Structure-activity relationships for 2-substituted imidazoles as alpha 2-adrenoceptor antagonists. J Med Chem. 1982 Jun;25(6):666–670. doi: 10.1021/jm00348a012. [DOI] [PubMed] [Google Scholar]
- Dabiré H., Dausse J. P., Mouillé P., Schmitt H., Meyer P. In vitro studies with (imidazolinyl-2)-2-benzodioxane-1-4 ((+/-)-170 150), a new potent alpha 2-adrenoceptor blocking agent. Eur J Pharmacol. 1982 Dec 17;86(1):87–90. doi: 10.1016/0014-2999(82)90401-0. [DOI] [PubMed] [Google Scholar]
- Docherty J. R., McGrath J. C. The actions of cirazoline on the rat vas deferens. Br J Pharmacol. 1984 May;82(1):9–14. doi: 10.1111/j.1476-5381.1984.tb16436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey J. C., Lane A. C., Roach A. G., Virdee N. K. Comparison of the alpha-adrenoceptor antagonist profiles of idazoxan (RX 781094), yohimbine, rauwolscine and corynanthine. Naunyn Schmiedebergs Arch Pharmacol. 1984 Feb;325(2):136–144. doi: 10.1007/BF00506193. [DOI] [PubMed] [Google Scholar]
- Doxey J. C., Roach A. G., Smith C. F. Studies on RX 781094: a selective, potent and specific antagonist of alpha 2-adrenoceptors. Br J Pharmacol. 1983 Mar;78(3):489–505. doi: 10.1111/j.1476-5381.1983.tb08809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew G. M. Pharmacological characterisation of the presynaptic alpha-adrenoceptor in the rat vas deferens. Eur J Pharmacol. 1977 Mar 21;42(2):123–130. doi: 10.1016/0014-2999(77)90351-x. [DOI] [PubMed] [Google Scholar]
- Duval N., Hicks P. E., Langer S. Z. Inhibitory effects of alpha, beta-methylene ATP on nerve-mediated contractions of the nictitating membrane in reserpinised cats. Eur J Pharmacol. 1985 Apr 16;110(3):373–377. doi: 10.1016/0014-2999(85)90567-9. [DOI] [PubMed] [Google Scholar]
- Hellewell P. G., Pearson J. D. Effect of sulphasalazine on pulmonary inactivation of prostaglandin F2 alpha in the pig. Br J Pharmacol. 1982 Jun;76(2):319–326. doi: 10.1111/j.1476-5381.1982.tb09223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks P. E., Cannon J. G. Cardiovascular effects of 2-(NN-dimethyl)amino-6,7-dihydroxy-1,2,3,4 tetrahydronaphthalene in pithed rats: differential antagonism by yohimbine and prazosin. J Pharm Pharmacol. 1980 Nov;32(11):786–788. doi: 10.1111/j.2042-7158.1980.tb13068.x. [DOI] [PubMed] [Google Scholar]
- Hyland L., Warnock P., Docherty J. R. An investigation of the actions of the calcium entry facilitator Bay K 8644 on the rat vas deferens. Eur J Pharmacol. 1984 Sep 17;104(3-4):363–367. doi: 10.1016/0014-2999(84)90414-x. [DOI] [PubMed] [Google Scholar]
- Langer S. Z., Dubocovich M. L. Cocaine and amphetamine antagonize the decrease of noradrenergic neurotransmission elicited by oxymetazoline but potentiate the inhibition by alpha-methylnorepinephrine in the perfused cat spleen. J Pharmacol Exp Ther. 1981 Jan;216(1):162–171. [PubMed] [Google Scholar]
- Langer S. Z., Pinto J. E. Possible involvement of a transmitter different from norepinephrine in the residual responses to nerve stimulation of the cat nictitating membrane after pretreatment with reserpine. J Pharmacol Exp Ther. 1976 Mar;196(3):697–713. [PubMed] [Google Scholar]
- MacDonald A., McGrath J. C. The distribution of adrenoceptors and other drug receptors between the two ends of the rat vas deferens as revealed by selective agonists and antagonists. Br J Pharmacol. 1980;71(2):445–458. doi: 10.1111/j.1476-5381.1980.tb10957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. C. Adrenergic and 'non-adrenergic' components in the contractile response of the vas deferens to a single indirect stimulus. J Physiol. 1978 Oct;283:23–39. doi: 10.1113/jphysiol.1978.sp012486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum L. A., Burnstock G. Evidence that ATP acts as a co-transmitter with noradrenaline in sympathetic nerves supplying the guinea-pig vas deferens. Eur J Pharmacol. 1983 Aug 19;92(1-2):161–163. doi: 10.1016/0014-2999(83)90126-7. [DOI] [PubMed] [Google Scholar]
- Pelayo F., Dubocovich M. L., Langer S. Z. Inhibition of neuronal uptake reduces the presynaptic effects of clonidine but not of alpha-methylnoradrenaline on the stimulation-evoked release of 3H-noradrenaline from rat occipital cortex slices. Eur J Pharmacol. 1980 Jun 13;64(2-3):143–155. doi: 10.1016/0014-2999(80)90037-0. [DOI] [PubMed] [Google Scholar]
- Ruffolo R. R., Jr, Rice P. J., Patil P. N., Hamada A., Miller D. D. Differences in the applicability of the easson-stedman hypothesis to the alpha 1- and alpha 2-adrenergic effects of phenethylamines and imidazolines. Eur J Pharmacol. 1983 Jan 21;86(3-4):471–475. doi: 10.1016/0014-2999(83)90199-1. [DOI] [PubMed] [Google Scholar]
- Shepperson N. B., Purcell T., Massingham R., Langer S. Z. In vitro studies on 6-fluoronoradrenaline at several peripheral sympathetic neuroeffector junctions. Naunyn Schmiedebergs Arch Pharmacol. 1981 Aug;317(1):1–4. doi: 10.1007/BF00506247. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984 Apr 13;100(1):85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi E. S., Ludvig N., Rónai A. Z., Folly G. Dissociation of presynaptic alpha 2-adrenoceptors following prazosin administration: presynaptic effect of prazosin. Eur J Pharmacol. 1983 Nov 25;95(3-4):287–290. doi: 10.1016/0014-2999(83)90648-9. [DOI] [PubMed] [Google Scholar]
- Westerink B. H., Korf J. Rapid concurrent automated fluorimetric assay of noradrenaline, dopamine, 3,4-dihydroxyphenylacetic acid, homovanillic acid and 3-methoxytyramine in milligram amounts of nervous tissue after isolation on Sephadex G10. J Neurochem. 1977 Oct;29(4):697–706. doi: 10.1111/j.1471-4159.1977.tb07788.x. [DOI] [PubMed] [Google Scholar]


