Abstract
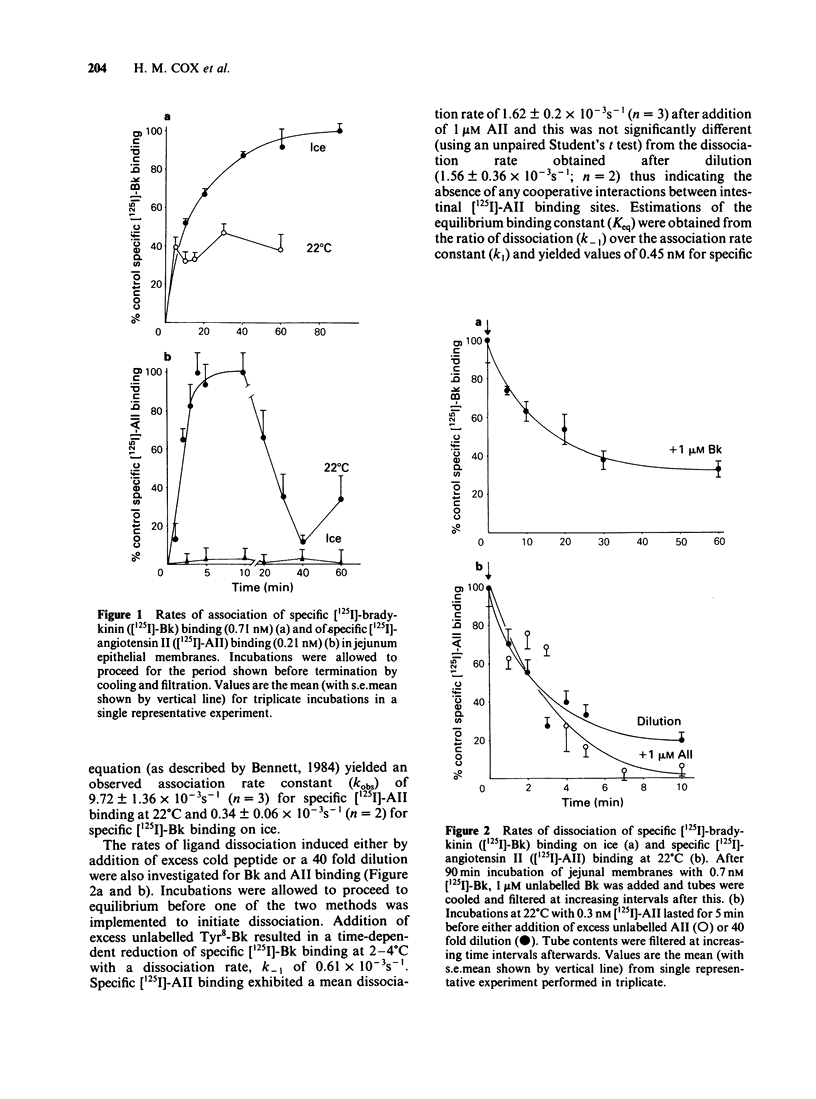
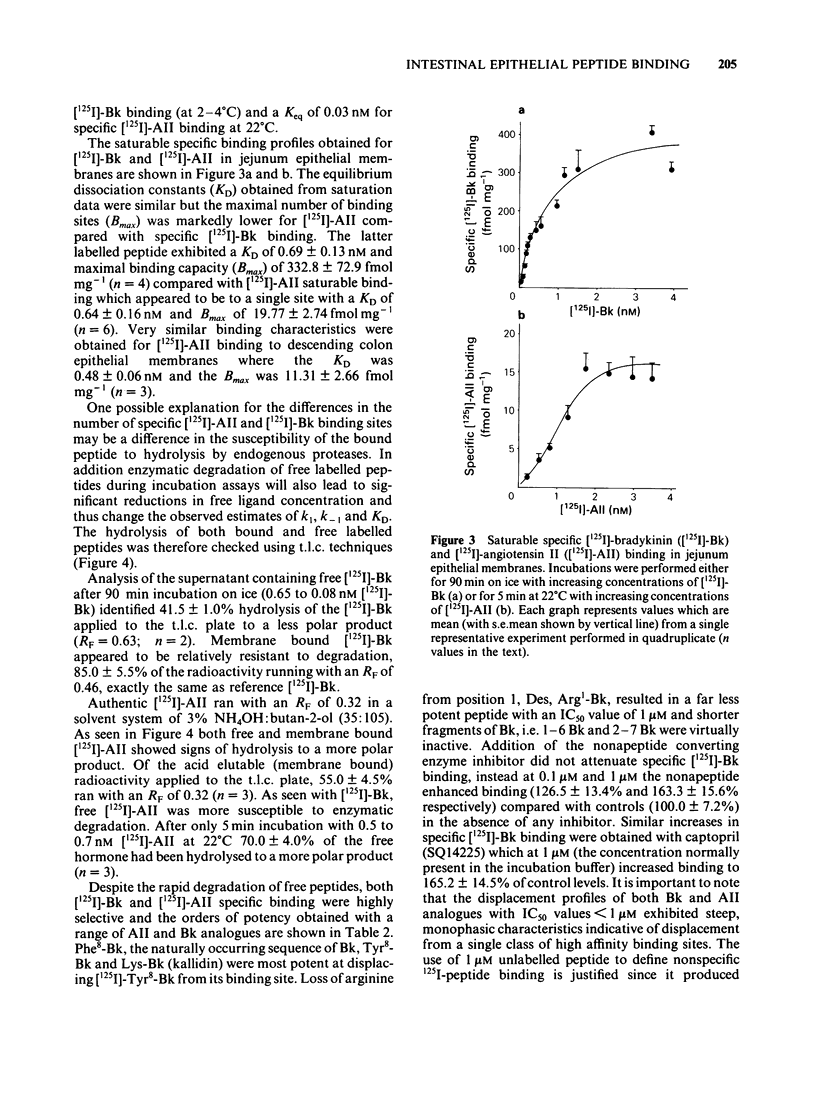
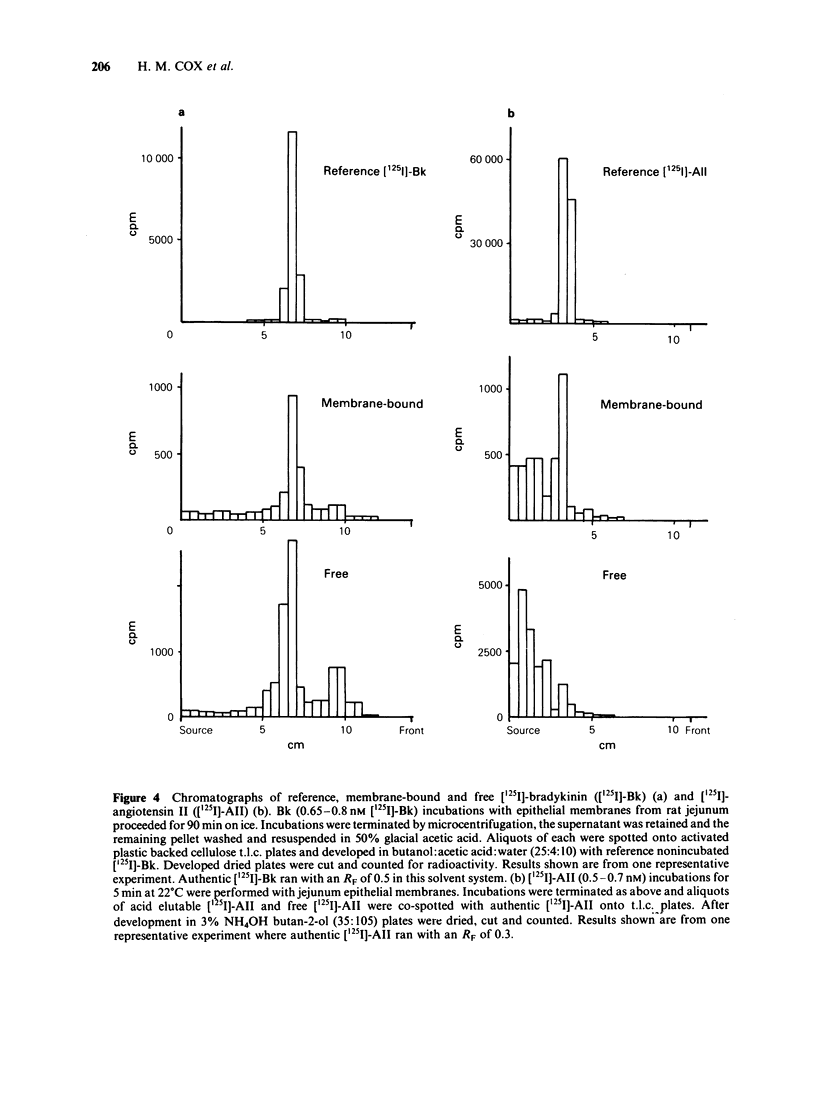
Specific [125I]-angiotensin II (AII) and [125I]-bradykinin (Bk) binding sites have been identified within epithelial membranes from rat jejunum and descending colon. These high affinity intestinal sites exhibited KD values of 0.64 +/- 0.16 nM for [125I]-AII and 0.69 +/- 0.13 nM for [125I]-Bk, which were similar to those for [125I]-AII (0.85 nM) and [125I]-Bk binding sites (1.03 nM) previously identified in renal cortex epithelia. Specific [125I]-AII binding capacity was only 19.77 +/- 2.74 fmol mg-1 in small intestine and 11.31 +/- 2.66 fmol mg-1 in descending colon epithelia while a larger population, 332.0 +/- 72.9 fmol-mg-1 of specific [125I]-Bk sites were identified in epithelial membranes from small intestine. Significant hydrolysis of both free [125I]-AII and [125I]-Bk was observed while membrane bound peptides remained relatively resistant to degradation. Whilst no corrections have been made to the observed values of KD and Bmax quoted above, one may assume that the calculated reductions in the free hormone concentration will result in a decrease of the KD value for both peptides. Loss of membrane bound peptide, particularly of [125I]-AII, may indicate that the calculated Bmax value is an underestimation. Despite the rapid degradation of unbound [125I]-AII and [125I]-Bk during incubations the kinetics of specific peptide binding were reversible and highly selective. The order of potency for specific [125I]-AII binding was [Sar1, Leu8]-AII greater than or equal to [Sar1, Thr8]-AII greater than or equal to AII greater than [Sar1, Ile8]-AII greater than or equal to [Des, Asp1, Ile8] AII greater than AIII. Specific [125I]-Bk binding was also highly selective, the order of potency being Phe8-Bk greater than or equal to Tyr8-Bk greater than or equal to Lys-Bk much greater than Des, Arg1-Bk. AII exhibited an IC50 of greater than 1mM for specific [125I]-Bk binding and likewise Phe8-Bk for specific [125I]-AII binding.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown G. P., Douglas J. G. Angiotensin II-binding sites in rat and primate isolated renal tubular basolateral membranes. Endocrinology. 1983 Jun;112(6):2007–2014. doi: 10.1210/endo-112-6-2007. [DOI] [PubMed] [Google Scholar]
- Brunton J., Parsons B. J., Poat J. A. Possible involvement of noradrenaline in the response of rat kidney cortex slices to angiotensin [proceedings]. J Physiol. 1978 Nov;284:73P–74P. [PubMed] [Google Scholar]
- Cornish-Bowden A., Koshland D. E., Jr Diagnostic uses of the Hill (Logit and Nernst) plots. J Mol Biol. 1975 Jun 25;95(2):201–212. doi: 10.1016/0022-2836(75)90390-3. [DOI] [PubMed] [Google Scholar]
- Cox H. M., Munday K. A., Poat J. A. Location of [125I]-angiotensin II receptors on rat kidney cortex epithelial cells. Br J Pharmacol. 1984 Aug;82(4):891–895. doi: 10.1111/j.1476-5381.1984.tb16487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox H. M., Munday K. A., Poat J. A. The binding of [125I]-angiotensin to rat renal epithelial cell membranes. Br J Pharmacol. 1983 May;79(1):63–70. doi: 10.1111/j.1476-5381.1983.tb10496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Halushka P. V., Margolius H. S., Spayne J. A. Mediators of the secretory response to kinins. Br J Pharmacol. 1984 Jul;82(3):597–607. doi: 10.1111/j.1476-5381.1984.tb10798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Margolius H. S. Kinins stimulate net chloride secretion by the rat colon. Br J Pharmacol. 1982 Apr;75(4):587–598. doi: 10.1111/j.1476-5381.1982.tb09178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N. T., Munday K. A., Parsons B. J. The effect of angiotensin on rat intestinal fluid transfer. J Endocrinol. 1970 Sep;48(1):39–46. doi: 10.1677/joe.0.0480039. [DOI] [PubMed] [Google Scholar]
- Gaginella T. S., Rimele T. J., Wietecha M. Studies on rat intestinal epithelial cell receptors for serotonin and opiates. J Physiol. 1983 Feb;335:101–111. doi: 10.1113/jphysiol.1983.sp014522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossmann H., Baukal A., Aguilera G., Catt K. J. Radioligand assay for angiotensin II receptors. Methods Enzymol. 1985;109:110–126. doi: 10.1016/0076-6879(85)09080-2. [DOI] [PubMed] [Google Scholar]
- Goodfriend T. L., Simpson R. U. Angiotensin receptors in bovine umbilical artery and their inhibition by nonsteroidal anti-inflammatory drugs. Br J Pharmacol. 1981 Feb;72(2):247–255. doi: 10.1111/j.1476-5381.1981.tb09121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis R. B., Manning D. C., Stewart J. M., Snyder S. H. [3H]Bradykinin receptor binding in mammalian tissue membranes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2630–2634. doi: 10.1073/pnas.78.4.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens N. R., Peach M. J., Carey R. M. Interactions between angiotensin peptides and the sympathetic nervous system mediating intestinal sodium and water absorption in the rat. J Clin Invest. 1981 Apr;67(4):1197–1207. doi: 10.1172/JCI110135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens N. R., Peach M. J., Carey R. M., Poat J. A., Munday K. A. Response of rat jejunum to angiotensin II: role of norepinephrine and prostaglandins. Am J Physiol. 1981 Jan;240(1):G17–G24. doi: 10.1152/ajpgi.1981.240.1.G17. [DOI] [PubMed] [Google Scholar]
- Manning D. C., Snyder S. H., Kachur J. F., Miller R. J., Field M. Bradykinin receptor-mediated chloride secretion in intestinal function. Nature. 1982 Sep 16;299(5880):256–259. doi: 10.1038/299256a0. [DOI] [PubMed] [Google Scholar]
- Musch M. W., Kachur J. F., Miller R. J., Field M., Stoff J. S. Bradykinin-stimulated electrolyte secretion in rabbit and guinea pig intestine. Involvement of arachidonic acid metabolites. J Clin Invest. 1983 May;71(5):1073–1083. doi: 10.1172/JCI110857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultzberg M., Hökfelt T., Nilsson G., Terenius L., Rehfeld J. F., Brown M., Elde R., Goldstein M., Said S. Distribution of peptide- and catecholamine-containing neurons in the gastro-intestinal tract of rat and guinea-pig: immunohistochemical studies with antisera to substance P, vasoactive intestinal polypeptide, enkephalins, somatostatin, gastrin/cholecystokinin, neurotensin and dopamine beta-hydroxylase. Neuroscience. 1980;5(4):689–744. doi: 10.1016/0306-4522(80)90166-9. [DOI] [PubMed] [Google Scholar]
- Schuster V. L., Kokko J. P., Jacobson H. R. Interactions of lysyl-bradykinin and antidiuretic hormone in the rabbit cortical collecting tubule. J Clin Invest. 1984 Jun;73(6):1659–1667. doi: 10.1172/JCI111372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K., Pisano J. J. Binding of [3H]bradykinin in isolated nephron segments of the rabbit. Am J Physiol. 1984 May;246(5 Pt 2):F732–F737. doi: 10.1152/ajprenal.1984.246.5.F732. [DOI] [PubMed] [Google Scholar]
- Turner A. J., Matsas R., Kenny A. J. Are there neuropeptide-specific peptidases? Biochem Pharmacol. 1985 May 1;34(9):1347–1356. doi: 10.1016/0006-2952(85)90669-0. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


